Spanish consensus on the diagnosis and management of adrenocortical carcinoma
- PMID: 40215284
- PMCID: PMC12053981
- DOI: 10.1530/ERC-25-0034
Spanish consensus on the diagnosis and management of adrenocortical carcinoma
Abstract
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with an estimated incidence of 0.7-2 cases per million/year. The rarity of this disease, coupled with limited preclinical models and clinical trials, has hindered progress, resulting in poor outcomes, with a 5-year survival rate of approximately 35%. Currently, the only available curative treatment is complete surgical resection of the adrenal tumor. For unresectable or metastatic ACC, the current standard therapeutic modalities are mitotane, chemotherapy, radiotherapy and locoregional treatments; however, these are noncurative. Mitotane has an adrenolytic and anti-steroidogenic effect, and it is used in the adjuvant setting for high-risk patients, as systemic therapy for metastatic disease, and/or to control hormonal secretion. While key pathways in ACC pathogenesis have been identified as potential therapeutic targets, results with targeted therapies remain modest, showing that there is a clinical unmet need for novel treatments or new combinations of exiting drugs. Effective management requires a multidisciplinary team of experts to optimize outcomes for patients. This article presents a multidisciplinary consensus on the diagnosis, management, prognosis and follow-up of patients with ACC, and the approach to two special contexts, ACC in pregnant women and hormone-producing ACC. The consensus was coordinated by the Spanish Society of Endocrinology and Nutrition (SEEN) and the Spanish Group of Neuroendocrine and Endocrine Tumors (GETNE), with contribution from experts from related societies including the Spanish Association of Surgeons (AEC), Spanish Society of Urology (AEU), Anatomic-Pathology (SEAP), Nuclear Medicine (SEMNIM), Medical Oncology (SEOM) and Radiotherapeutic Oncology (SEOR).
Keywords: ENSAT; adrenal tumor; adrenalectomy; adrenocortical carcinoma; mitotane.
Conflict of interest statement
MAC received speakers’ honoraria and consulting fees from Esteve and Recordati Rare Diseases. ACB received payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing, educational events or participation on a data safety monitoring board or advisory board from Astellas, AstraZeneca, BMS, Lilly, MSD, Ipsen, Novartis and Esteve. PJF received honoraria for speakers' bureau participation, and serving on advisory boards from Astellas, AstraZeneca, Bristol-Myers Squibb (BMS), Esteve, Merck Sharp & Dohme (MSD), Novartis, Nutricia, Pfizer, Rovi, Takeda and Viatris and research grants from Astellas, AstraZeneca, BMS and MSD. MRF received honoraria for lectures, manuscript writings, educational events or advisory boards from Telix, Sirtex Medical , Janssen, Novartis and Astellas. FAH received honoraria for lectures and advisor board from HRA Pharma and Recordatti. JLVC received honoraria for lectures, traveling, educational events or advisory boards from Novartis, Sirtex Medical and Terumo. The authors declare no potential conflict of interest.
Figures
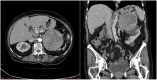


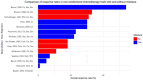


References
-
- Abraham J, Bakke S, Rutt A, et al. . 2002. A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. Cancer 94 2333–2343. (10.1002/CNCR.10487) - DOI - PubMed

