Control of morphogenesis during the Staphylococcus aureus cell cycle
- PMID: 40215301
- PMCID: PMC11988411
- DOI: 10.1126/sciadv.adr5011
Control of morphogenesis during the Staphylococcus aureus cell cycle
Abstract
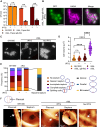
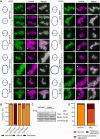
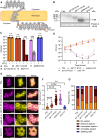
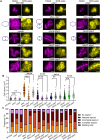
Bacterial cell division is a complex, multistage process requiring septum development while maintaining cell wall integrity. A dynamic, macromolecular protein complex, the divisome, tightly controls morphogenesis both spatially and temporally, but the mechanisms that tune septal progression are largely unknown. By studying conditional mutants of genes encoding DivIB, DivIC, and FtsL, an essential trimeric complex central to cell division in bacteria, we demonstrate that FtsL and DivIB play independent, hierarchical roles coordinating peptidoglycan synthesis across specific septal developmental checkpoints. They are required for the localization of downstream divisome components and the redistribution of peptidoglycan synthesis from the cell periphery to the septum. This is achieved by positive regulation of septum production and negative regulation of peripheral cell wall synthesis. Our analysis has led to a model for the coordination of cell division in Staphylococcus aureus, forming a framework for understanding how protein localization and function are integrated with cell wall structural dynamics across the bacteria.
Figures

References
-
- Pinho M. G., Kjos M., Veening J.-W., How to get (a)round: Mechanisms controlling growth and division of coccoid bacteria. Nat. Rev. Microbiol. 11, 601–614 (2013). - PubMed
-
- Buddelmeijer N., Beckwith J., A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 52, 1315–1327 (2004). - PubMed
-
- Noirclerc-Savoye M., Le Gouëllec A., Morlot C., Dideberg O., Vernet T., Zapun A., In vitro reconstitution of a trimeric complex of DivIB, DivIC and FtsL, and their transient co-localization at the division site in Streptococcus pneumoniae. Mol. Microbiol. 55, 413–424 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

