Discovery of synthetic small molecules targeting the central regulator of Salmonella pathogenicity
- PMID: 40215303
- PMCID: PMC11988454
- DOI: 10.1126/sciadv.adr5235
Discovery of synthetic small molecules targeting the central regulator of Salmonella pathogenicity
Abstract
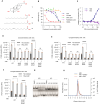
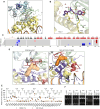
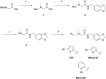
The enteric pathogen Salmonella enterica serovar Typhimurium relies on the activity of effector proteins to invade, replicate, and disseminate into host epithelial cells and other tissues, thereby causing disease. Secretion and injection of effector proteins into host cells is mediated by dedicated secretion systems, which hence represent major virulence determinants. Here, we report the identification of a synthetic small molecule with drug-like properties, C26, which suppresses the secretion of effector proteins and consequently hinders bacterial invasion of eukaryotic cells. C26 binds to and inhibits HilD, the transcriptional regulator of the major secretion systems. Although sharing the same binding pocket as the previously described long-chain fatty acid ligands, C26 inhibits HilD with a unique binding mode and a distinct mechanism. We provide evidence of intramacrophage activity and present analogs with improved potency and suitability as scaffolds to develop antivirulence agents against Salmonella infections in humans and animals.
Figures






References
-
- Theuretzbacher U., Piddock L. J. V., Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe 26, 61–72 (2019). - PubMed
-
- Allen R. C., Popat R., Diggle S. P., Brown S. P., Targeting virulence: Can we make evolution-proof drugs? Nat. Rev. Microbiol. 12, 300–308 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

