Female sex hormones exacerbate retinal neurodegeneration
- PMID: 40215317
- PMCID: PMC11988432
- DOI: 10.1126/sciadv.adr6211
Female sex hormones exacerbate retinal neurodegeneration
Abstract
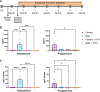
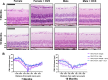
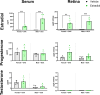
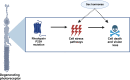
Neurodegenerative disorders such as Alzheimer's disease and macular degeneration represent major sources of human suffering, yet factors influencing disease severity remain poorly understood. Sex has been implicated as one modifying factor. Here, we show that female sex is a risk factor for worsened outcomes in a model of retinal degeneration and that this susceptibility is caused by the presence of female-specific sex hormones. The adverse effect of female sex hormones was specific to diseased retinal neurons, and depletion of these hormones ameliorated this phenotypic effect, while reintroduction worsened rates of disease in females. Transcriptional analysis of retinas showed significant differences between genes involved in pyroptosis, inflammatory responses, and endoplasmic reticulum stress-induced apoptosis between males and females with retinal degeneration. These findings provide crucial insights into the pathogenesis of neurodegenerative diseases and how sex hormones can affect disease severity. These findings have far-reaching implications for clinical trial design and the use of hormonal therapy in females with certain neurodegenerative disorders.
Figures




Update of
-
Female sex hormones exacerbate retinal neurodegeneration.bioRxiv [Preprint]. 2024 Jul 16:2024.07.11.603104. doi: 10.1101/2024.07.11.603104. bioRxiv. 2024. Update in: Sci Adv. 2025 Apr 11;11(15):eadr6211. doi: 10.1126/sciadv.adr6211. PMID: 39071341 Free PMC article. Updated. Preprint.
References
-
- Marin A. I., Poppelaars F., Wagner B. D., Palestine A. G., Patnaik J. L., Holers V. M., Frazer-Abel A. A., Mathias M. T., Manoharan N., Fonteh C. N., Mandava N., Lynch A. M., University of Colorado Retina Research Group , Sex and age-related differences in complement factors among patients with intermediate age-related macular degeneration. Transl. Vis. Sci. Technol. 11, 22 (2022). - PMC - PubMed
-
- Sasaki M., Harada S., Kawasaki Y., Watanabe M., Ito H., Tanaka H., Takeuchi A., Tsubota K., Takebayashi T., Nishiwaki Y., Kawasaki R., Gender-specific association of early age-related macular degeneration with systemic and genetic factors in a Japanese population. Sci. Rep. 8, 785 (2018). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

