Generation of an inflammatory niche in a hydrogel depot through recruitment of key immune cells improves efficacy of mRNA vaccines
- PMID: 40215318
- PMCID: PMC11988412
- DOI: 10.1126/sciadv.adr2631
Generation of an inflammatory niche in a hydrogel depot through recruitment of key immune cells improves efficacy of mRNA vaccines
Abstract
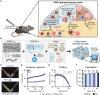
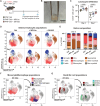
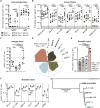
Messenger RNA (mRNA) delivered in lipid nanoparticles (LNPs) rose to the forefront of vaccine candidates during the COVID-19 pandemic due to scalability, adaptability, and potency. Yet, there remain critical areas for improvements of these vaccines in durability and breadth of humoral responses. In this work, we explore a modular strategy to target mRNA/LNPs to antigen-presenting cells with an injectable polymer-nanoparticle (PNP) hydrogel technology, which recruits key immune cells and forms an immunological niche in vivo. We characterize this niche on a single-cell level and find it is highly tunable through incorporation of adjuvants like MPLAs and 3M-052. Delivering commercially available severe acute respiratory syndrome coronavirus 2 mRNA vaccines in PNP hydrogels improves the durability and quality of germinal center reactions, and the magnitude, breadth, and durability of humoral responses. The tunable immune niche formed within PNP hydrogels effectively skews immune responses based on encapsulated adjuvants, creating opportunities to precisely modulate mRNA/LNP vaccines for various indications from infectious diseases to cancers.
Figures



Update of
-
Generation of an inflammatory niche in an injectable hydrogel depot through recruitment of key immune cells improves efficacy of mRNA vaccines.bioRxiv [Preprint]. 2024 Dec 20:2024.07.05.602305. doi: 10.1101/2024.07.05.602305. bioRxiv. 2024. Update in: Sci Adv. 2025 Apr 11;11(15):eadr2631. doi: 10.1126/sciadv.adr2631. PMID: 39026835 Free PMC article. Updated. Preprint.
Similar articles
-
Generation of an inflammatory niche in an injectable hydrogel depot through recruitment of key immune cells improves efficacy of mRNA vaccines.bioRxiv [Preprint]. 2024 Dec 20:2024.07.05.602305. doi: 10.1101/2024.07.05.602305. bioRxiv. 2024. Update in: Sci Adv. 2025 Apr 11;11(15):eadr2631. doi: 10.1126/sciadv.adr2631. PMID: 39026835 Free PMC article. Updated. Preprint.
-
SMART-lipid nanoparticles enabled mRNA vaccine elicits cross-reactive humoral responses against the omicron sub-variants.Mol Ther. 2024 May 1;32(5):1284-1297. doi: 10.1016/j.ymthe.2024.02.028. Epub 2024 Feb 27. Mol Ther. 2024. PMID: 38414245 Free PMC article.
-
Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses.Immunity. 2021 Dec 14;54(12):2877-2892.e7. doi: 10.1016/j.immuni.2021.11.001. Epub 2021 Nov 4. Immunity. 2021. PMID: 34852217 Free PMC article.
-
mRNA vaccines: miRNA-based controlled biodistribution and directed adjuvantation.Trends Immunol. 2025 May;46(5):357-360. doi: 10.1016/j.it.2025.03.004. Epub 2025 Apr 22. Trends Immunol. 2025. PMID: 40268657 Review.
-
mRNA Vaccines: Design Principles, Mechanisms, and Manufacturing-Insights From COVID-19 as a Model for Combating Infectious Diseases.Biotechnol J. 2025 Feb;20(2):e202400596. doi: 10.1002/biot.202400596. Biotechnol J. 2025. PMID: 39989260 Review.
Cited by
-
Hydrogels in Veterinary Vaccine Development: Types, Mechanisms, and Applications.Gels. 2025 Jun 18;11(6):468. doi: 10.3390/gels11060468. Gels. 2025. PMID: 40558766 Free PMC article. Review.
-
Polymer oxidation: A strategy for the controlled degradation of injectable cryogels.Mater Today Bio. 2025 Apr 8;32:101743. doi: 10.1016/j.mtbio.2025.101743. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40343169 Free PMC article.
-
Unlock the sustained therapeutic efficacy of mRNA.J Control Release. 2025 Jul 10;383:113837. doi: 10.1016/j.jconrel.2025.113837. Epub 2025 May 12. J Control Release. 2025. PMID: 40368188 Review.
-
Fusion of Complement Fragment C3d Enhances Germinal Center Responses to HIV-1 Envelope Glycoproteins.bioRxiv [Preprint]. 2025 Jul 1:2025.06.28.661730. doi: 10.1101/2025.06.28.661730. bioRxiv. 2025. PMID: 40631158 Free PMC article. Preprint.
References
-
- Pardi N., Hogan M. J., Weissman D., Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 65, 14–20 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

