Particulate matter exposure induces pulmonary TH2 responses and oxidative stress-mediated NRF2 activation in mice
- PMID: 40215613
- PMCID: PMC12018062
- DOI: 10.1016/j.redox.2025.103632
Particulate matter exposure induces pulmonary TH2 responses and oxidative stress-mediated NRF2 activation in mice
Abstract
Introduction: Particulate matter (PM) is a harmful air pollutant associated with respiratory and cardiovascular diseases, but its effects on adaptive immunity are poorly understood.
Objectives: This study investigates the role of NRF2 in T cells in mediating immune and pulmonary responses to long-term PM exposure, highlighting its impact on inhalation toxicity.
Methods: To establish a mouse model of lung injury induced by PM exposure, C57BL/6 mice were intranasally administered 20 μg/kg PM10 or PM2.5 daily for 16 weeks. Lung injury parameters were analyzed in bronchoalveolar lavage fluid (BALF), plasma, and lung tissue. Changes in the proportion of immune cells in the lymph nodes and spleen were analyzed.
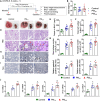
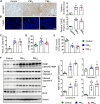
Results: Mice exposed to PM for 16 weeks showed severe lung damage, such as inflammatory cell infiltration, thickened alveolar walls, and increased oxidative stress and apoptosis. PM exposure also increased collagen and fibronectin levels, indicating tissue remodeling. Immune cell analysis revealed reduced B cell expansion, increased IL-4-producing CD4+ T cells, and decreased IFN-γ- and TNF-α-producing CD4+ T cells, accompanied by higher TH2 cytokines and plasma IgE and IgG1 levels. PM activated the NRF2 pathway, skewing immune responses toward TH2 differentiation, which worsened lung inflammation.
Conclusions: These findings highlight how PM exposure disrupts immune balance and exacerbates conditions like asthma and chronic obstructive pulmonary disease by promoting TH2-driven inflammation through NRF2 activation.
Keywords: NRF2; Oxidative stress; Particulate matter; Th2 immunity.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have declared no conflict of interest.
Figures






Similar articles
-
Unveiling the potent effect of vitamin D: harnessing Nrf2/HO-1 signaling pathways as molecular targets to alleviate urban particulate matter-induced asthma inflammation.BMC Pulm Med. 2024 Jan 25;24(1):55. doi: 10.1186/s12890-024-02869-2. BMC Pulm Med. 2024. PMID: 38273268 Free PMC article.
-
Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter.J Immunol. 2008 Oct 1;181(7):4545-59. doi: 10.4049/jimmunol.181.7.4545. J Immunol. 2008. PMID: 18802057 Free PMC article.
-
The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice.PLoS One. 2017 Feb 28;12(2):e0173158. doi: 10.1371/journal.pone.0173158. eCollection 2017. PLoS One. 2017. PMID: 28245275 Free PMC article.
-
The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles.Free Radic Biol Med. 2008 May 1;44(9):1689-99. doi: 10.1016/j.freeradbiomed.2008.01.028. Epub 2008 Feb 13. Free Radic Biol Med. 2008. PMID: 18313407 Free PMC article. Review.
-
Particulate matter, oxidative stress and neurotoxicity.Neurotoxicology. 2008 May;29(3):479-88. doi: 10.1016/j.neuro.2007.12.004. Epub 2008 Jan 4. Neurotoxicology. 2008. PMID: 18289684 Review.
References
-
- WHO Guidelines Approved by the Guidelines Review Committee . World Health Organization© World Health Organization; Geneva: 2021. WHO Global Air Quality Guidelines: Particulate Matter (PM(25) and PM(10)), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. 2021. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

