Intranasal kisspeptin administration rapidly stimulates gonadotropin release in humans
- PMID: 40215751
- PMCID: PMC12018048
- DOI: 10.1016/j.ebiom.2025.105689
Intranasal kisspeptin administration rapidly stimulates gonadotropin release in humans
Abstract
Background: Kisspeptin administration by intravenous or subcutaneous routes activates hypothalamic gonadotropin-releasing hormone (GnRH) neurons and is being developed to treat reproductive disorders. However, these invasive routes markedly limit patient acceptability and clinical use. Recent rodent data has identified a large GnRH population within the olfactory system communicating directly with hypothalamic GnRH neurons. Intranasal kisspeptin administration may be able to capitalise on this novel pathway and thus offer a potential non-invasive approach to stimulate reproductive hormones. Herein, we examine intranasal kisspeptin using human, pharmaceutical, and rodent studies.
Methods: Reproductive hormone profiles were measured after intranasal kisspeptin administration in healthy volunteers and patients with reproductive disorders as part of a randomised, double-blinded, crossover, placebo-controlled clinical study. Pharmaceutical testing evaluated the chemical stability and nasal kisspeptin delivery, and rodent studies provided mechanistic insight.
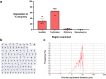
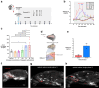
Findings: Intranasal kisspeptin-54 rapidly stimulates gonadotropin release in healthy men and women, and in patients with a common reproductive disorder (hypothalamic amenorrhoea), without any side effects or adverse events encountered. Specifically, intranasal kisspeptin (at 12.8 nmol/kg) induced clinically-significant mean maximal increases above baseline in serum luteinising hormone in all study groups: 4.4 ± 0.6 IU/L (mean difference = 3.1 IU/L [95% CI, 1.2-4.9], P = 0.002 vs. placebo) in healthy men; 1.4 ± 0.3 IU/L (mean difference = 1.0 IU/L [95% CI, 0.4-1.7], P = 0.004 vs. placebo) in healthy women; 4.4 ± 0.2 IU/L (mean difference = 4.3 IU/L [95% CI, 2.7-6.0], P < 0.001 vs. placebo) in patients with hypothalamic amenorrhoea. Kisspeptin-54 was delivered effectively via nasal spray and was stable for up to 60 days at 4 °C. Mirroring the human effects, intranasal kisspeptin-54 in adult C57BL/6J male mice stimulates luteinising hormone release. Further mechanistic insights reveal the accumulation of fluorescently-tagged kisspeptin in the olfactory epithelium, as well as the presence of kisspeptin receptors in olfactory bulb GnRH neurons, implicating the involvement of these extra-hypothalamic GnRH neurons in the pathway mediating intranasal kisspeptin's effects on reproductive hormones.
Interpretation: We demonstrate the clinical potential for intranasal kisspeptin delivery as the first non-invasive method to robustly and safely stimulate gonadotropins with kisspeptin and potentially transform the management of reproductive disorders.
Funding: National Institute for Health and Care Research (NIHR)/NIHR Imperial Biomedical Research Centre/Medical Research Council (MRC).
Keywords: Fertility; Kisspeptin; Neuroendocrinology; Neuropeptides; Reproduction.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests AA has conducted consultancy work for Myovant Sciences Ltd outside the submitted work. WSD has conducted consultancy work for AbCellera, PostEra, KaNDy Therapeutics Ltd, and Myovant Sciences Ltd outside the submitted work. The other authors declare no conflict-of-interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

