Inflammatory and clinical risk factors for asthma attacks (ORACLE2): a patient-level meta-analysis of control groups of 22 randomised trials
- PMID: 40215991
- PMCID: PMC12117016
- DOI: 10.1016/S2213-2600(25)00037-2
Inflammatory and clinical risk factors for asthma attacks (ORACLE2): a patient-level meta-analysis of control groups of 22 randomised trials
Abstract
Background: Clinical risk factors for severe asthma attacks have been identified, but their incremental prognostic values are unclear. Additionally, the incremental contribution of type 2 inflammation, a common, treatable process, is undetermined. We aimed to quantify the prognostic value of baseline characteristics and type 2 inflammatory biomarkers, specifically blood eosinophil count and fractional exhaled nitric oxide (FeNO), to predict asthma attacks.
Methods: In this systematic review and meta-analysis of randomised controlled trials (RCTs), Oxford Asthma Attack Risk Scale 2 (ORACLE2), we searched MEDLINE from Jan 1, 1993, to April 1, 2021, for trials investigating fixed treatment regimen effects on asthma attack rates for at least 6 months with baseline blood eosinophil count and FeNO. Eligible participants were aged 12 years or older with asthma (any severity) who had been randomly assigned to the control group of an RCT. Relevant trials were manually retrieved and reviewed by two independent reviewers (SC and IDP). Disagreements were discussed with five reviewers. Individual patient data (IPD) for meta-analysis were requested from study authors. We investigated the rate of severe asthma attacks (≥3 days of systemic corticosteroids) for at least 6 months and prognostic effects of baseline blood eosinophil count and FeNO in control group participants. Rate ratios (RRs) with 95% CIs were derived for annualised asthma attack rates from negative binomial models adjusted for key variables, including blood eosinophil count and FeNO, and interactions between these type 2 inflammatory biomarkers were explored. Certainty of evidence was assessed using GRADE. The heterogeneity of the included studies and potential for ecological bias were quantified by the concordance statistic (C-statistic). This study was registered with PROSPERO, CRD42021245337.
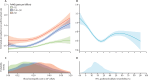
Findings: We identified 976 potentially eligible studies. After automated screening, we manually reviewed 219 full-text articles. Of these, 19 publications comprising 23 RCTs were eligible. 6513 participants (4140 [64%] female; 2370 [36%] male; three missing) spanning 22 RCTs were included for data analysis. 5972 (92%) of 6513 patients had moderate-to-severe asthma. 4615 asthma attacks occurred during 5482 person-years of follow-up (annualised rate 0·84 per person-year). Higher blood eosinophil count or FeNO was linked to higher asthma attack risk (per 10-fold increase, RR 1·48 [95% CI 1·30-1·68] for blood eosinophil count and 1·44 [1·26-1·65] for FeNO; high-certainty evidence). Other prognostic factors were attack history (yes vs no, RR 1·94 [1·61-2·32]); disease severity (severe vs moderate, RR 1·57 [1·22-2·03]); FEV1 percentage predicted (FEV1%; per 10% decrease, RR 1·11 [1·08-1·15]); and 5-item Asthma Control Questionnaire score (ACQ-5; per 0·5 increase, RR 1·10 [1·07-1·13]). High blood eosinophil count and FeNO combined were associated with greater risk than either prognostic factor separately. Bronchodilator reversibility was associated with lower risk of severe asthma attacks (per 10% increase, RR 0·93 [0·90-0·96]), with the reduction observed primarily between 0% and 25%. Regarding heterogeneity of the included studies, the C-statistic ranged from 0·58 to 0·95, indicating major differences in patient and disease characteristics between studies. In the univariable meta-analysis per trial, we found substantial heterogeneity in associations between studies, with I2 statistics ranging from 0·56 to 0·97.
Interpretation: Blood eosinophil count, FeNO, asthma attack history, disease severity, low lung function (low FEV1%), and symptoms (ACQ-5 score) are key predictors of asthma attacks. Conversely, we found that moderate bronchodilator reversibility was associated with reduced risk. These findings from high-quality multinational RCTs support incorporation of blood eosinophils and FeNO into clinical risk stratification for targeted risk reduction. More individualised clinical decision-making models should be explored.
Funding: National Institute of Health and Care Research Oxford Biomedical Research Centre; Association pulmonaire du Québec; Fonds de recherche du Québec-Santé; Québec Air-Intersectorialité-Respiratoire-Son network; Stichting Astma Bestrijding; Leiden University Fund; and Academy of Medical Sciences.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests Outside this work, CC-P has received speaker honoraria from AstraZeneca, GSK, and Sanofi-Regeneron; and consultancy fees from AstraZeneca, GSK, and Sanofi-Regeneron. SR has received salary support from the National Institute for Health and Care Research (NIHR) UK and the Charlie's Foundation for Research. SR also declares speaker fees from GSK and AstraZeneca, and conference travel support from AstraZeneca. MEW has received consulting, advisory, or speaking honoraria from Allakos, Amgen, Areteia Therapeutics, Arrowhead Pharmaceutical, AstraZeneca, Avalo Therapeutics, Celldex, Connect Biopharma, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Kymera, Merck, MyBiometry, Pharming, Phylaxis, Pulmatrix, Rapt Therapeutics, Recludix Pharma, Regeneron, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Uniquity Bio, Upstream Bio, Verona Pharma, and Zurabio. GB has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Merck Sharp & Dohme, Novartis, and Sanofi-Regeneron; he is President of the Belgian Respiratory Society. JC has received grants or contracts from Regeneron, Sanofi, and Novartis. He also has received consulting fees from AstraZeneca, Amgen, Regeneron, and Sanofi and payment of honoraria from AstraZeneca, Amgen, Regeneron, and Sanofi. SED has received consultancy fees from AstraZeneca. CEB has received grants and consultancy fees from 4D Pharma, Areteia, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche, and Sanofi. MC has received grants or contracts from American Lung Association, AstraZeneca, Gala Therapeutics, Genentech, GSK, National Institutes of Health, Nocion, Novartis, PCORI, Pulmatrix, Sanofi-Aventis, Shionogi, and Theravance Biopharma. He has also received consulting fees from Allakos, Amgen, Apogee, Apreo Health, Arrowhead Pharmaceuticals, Blueprint Medicines, Connect BioPharma, Evommune, Genentech, GSK, Jasper, Kinaset, Merck, Novartis, OM Pharma, Pfizer, Pioneering Medicines, Sanofi-Aventis, Teva, Third Rock Ventures, Upstream Bio, and Verona Pharmaceuticals; honoraria from Amgen, AstraZeneca, Med Learning Group, Regeneron Pharmaceuticals, and Sanofi; and stock options from Aer Therapeutics. NAH has received honoraria for serving as a consultant or advisor to GSK, AstraZeneca, Genentech, Sanofi, Regeneron, Verona, and Amgen; and research grant support from GSK, AstraZeneca, Genentech, Regeneron, and Sanofi. DJJ has received advisory board and speakers fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva, GSK, Sanofi-Regeneron, and Chiesi. NM is an employee and shareholder with AstraZeneca. AL is employed by AstraZeneca. ES is a former GSK employee and provided anonymised data from GSK studies CAPTAIN and DREAM; provided inputs into manuscript development; and holds GSK stock options. CC holds shares in GSK and is an employee of GSK. MEH is a Sanofi employee. CTJH is a former employee of Genentech. AS is a Novartis employee. TSCH was supported by a Wellcome Trust Fellowship (211050/Z/18/z); he reports grants from the Guardians of the Beit Fellowship, Pfizer, NIHR Oxford Biomedical Research Centre (BRC), University of Oxford, Kymab, Arcturis, and Asthma+Lung UK; and personal fees from AstraZeneca Pieris. RWB has received institutional research funding from AstraZeneca, Teva, Health Research Council, Cure Kidz, and Perpetual Guardian; personal fees from AstraZeneca, Avillion, and Teva; and is Chair of the Asthma Foundation of New Zealand adolescent and adult asthma guidelines, a reviewer for GINA, and a former member of the Global Initiative for Chronic Obstructive Lung Disease board. JKS has received non-restricted research grants from AstraZeneca, European Respiratory Society Severe Heterogeneous Asthma Research collaboration—Patient Centred Clinical Research Collaboration, Register of Adult Patients with Severe Asthma for Optimal Disease Management Foundation, and ZonMw. EWS has received consultancy fees from GSK. IDP has received honoraria for speaking at sponsored meetings from AstraZeneca, Circassia, AmgenNovartis, Chiesi, Sanofi-Regeneron, Menarini, and GSK; and payments for organising educational events from AstraZeneca, GSK, Sanofi-Regeneron, and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi-Regeneron, AstraZeneca, GSK, Novartis, Teva, Merck, Circassia, and Amgen. He has received sponsorship to attend international scientific meetings from GSK, AstraZeneca, Sanofi, and Regeneron. SC reports non-restricted research grants from NIHR Oxford BRC, the Quebec Respiratory Health Research Network, the Fondation Québécoise en Santé Respiratoire, AstraZeneca, Sanofi-Regeneron, and Circassia Niox group; speaker honoraria from AstraZeneca, GSK, Sanofi-Regeneron, and Valeo Pharma; consultancy fees from FirstThought, AstraZeneca, GSK, Sanofi-Regeneron, Access Biotechnology, and Access Industries; and sponsorship to attend or speak at international scientific meetings by or for AstraZeneca and Sanofi-Regeneron. He is an advisory board member for and holds stock options in Biometry—a company that is developing an exhaled nitric oxide device (myBiometry). He advised the Institut national d'excellence en santé et services sociaux on an update of the asthma general practice information booklet for general practitioners. Within the submitted work, CC-P has received an education scholarship from the Université de Sherbrooke, and SC reports that he has received non-restricted research grants from the Québec Air-Intersectorialité-Respiratoire-Son network, the Academy of Medical Sciences, and the NIHR Oxford BRC, is the holder of the Association pulmonaire du Québec's Research Chair in Respiratory medicine, and is a clinical research scholar of the Fonds de recherche du Québec. All other authors declare no competing interests.
Figures

References
-
- Global Initiative for Asthma Global strategy for asthma management and prevention. May 2024. https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Repo...
-
- Couillard S, Petousi N, Smigiel K, Molfino N. Towards a predict and prevent approach in obstructive airways diseases. J Allergy Clin Immunol Pract. 2023;11:704–712. - PubMed
-
- Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting β2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. - PubMed
-
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. - PubMed
-
- Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391:350–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

