Optogenetic control of pheromone gradients and mating behavior in budding yeast
- PMID: 40216553
- PMCID: PMC11992364
- DOI: 10.26508/lsa.202403078
Optogenetic control of pheromone gradients and mating behavior in budding yeast
Abstract
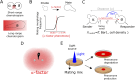
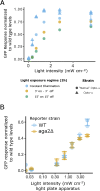
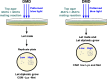
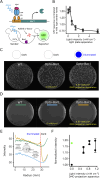
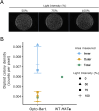
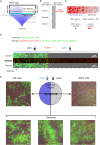
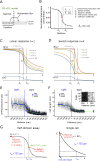
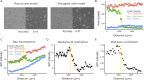
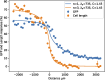
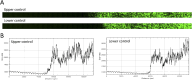
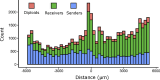
During mating in budding yeast, cells use pheromones to locate each other and fuse. This model system has shaped our current understanding of signal transduction and cell polarization in response to extracellular signals. The cell populations producing extracellular signal landscapes themselves are, however, less well understood, yet crucial for functionally testing quantitative models of cell polarization and for controlling cell behavior through bioengineering approaches. Here we engineered optogenetic control of pheromone landscapes in mating populations of budding yeast, hijacking the mating-pheromone pathway to achieve spatial control of growth, cell morphology, cell-cell fusion, and distance-dependent gene expression in response to light. Using our tool, we were able to spatially control and shape pheromone gradients, allowing the use of a biophysical model to infer the properties of large-scale gradients generated by mating populations in a single, quantitative experimental setup, predicting that the shape of such gradients depends quantitatively on population parameters. Spatial optogenetic control of diffusible signals and their degradation provides a controllable signaling environment for engineering artificial communication and cell-fate systems in gel-embedded cell populations without the need for physical manipulation.
© 2025 Banderas et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
