Myeloma mesenchymal stem cells' bioenergetics afford a novel selective therapeutic target
- PMID: 40216736
- PMCID: PMC11992228
- DOI: 10.1038/s41389-025-00554-5
Myeloma mesenchymal stem cells' bioenergetics afford a novel selective therapeutic target
Abstract
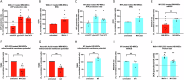
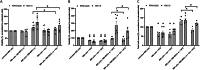
Bone-marrow mesenchymal stem cells (BM-MSCs) rely on glycolysis, yet their trafficked mitochondria benefit recipient cells' bioenergetics in regenerative and cancerous settings, most relevant to BM-resident multiple myeloma (MM) cells. Fission/fusion dynamics regulate mitochondria function. Proteomics demonstrates excessive mitochondrial processes in BM-MSCs from MM patients compared to normal donors (ND). Thus, we aimed to characterize BM-MSCs (ND, MM) mitochondrial fitness, bioenergetics and dynamics with a focus on therapeutics. MM-MSCs displayed compromised mitochondria evidenced by decreased mitochondrial membrane potential (ΔΨm) and elevated proton leak. This was accompanied by stimulation of stress-coping mechanisms: spare respiratory capacity (SRC), mitochondrial fusion and UPRmt. Interfering with BM-MSCs mitochondrial dynamics equilibrium demonstrated their significance to bioenergetics and fitness according to the source. While ND-MSCs depended on fission, reducing MM-MSCs fusion attenuated glycolysis, OXPHOS and mtROS. Interestingly, optimization of mtROS levels is central to ΔΨm preservation in MM-MSCs only. MM-MSCs also demonstrated STAT3 activation, which regulates their OXPHOS and SRC. Targeting MM-MSC' SRC with Venetoclax diminished their pro-MM support and sensitized co-cultured MM cells to Bortezomib. Overall, MM-MSCs distinct mitochondrial bioenergetics are integral to their robustness. Repurposing Venetoclax as anti-SRC treatment in combination with conventional anti-MM drugs presents a potential selective way to target MM-MSCs conferred drug resistance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All procedures were performed in compliance with relevant laws and institutional guidelines and have been approved by the Meir Medical Center Helsinki Committee (REF: 0205-12-MMC ; REF: 0045-11-MMC). All patients or their guardians/legally authorized representatives/next of kin provided written informed consent for participation in the study and the use of samples.
Figures


References
-
- Attar-Schneider O, Zismanov V, Dabbah M, Tartakover-Matalon S, Drucker L, Lishner M. Multiple myeloma and bone marrow mesenchymal stem cells’ crosstalk: effect on translation initiation. Mol Carcinog. 2016;55:1343–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

