PAX3 expression patterns in ocular surface melanocytes
- PMID: 40216818
- PMCID: PMC11992251
- DOI: 10.1038/s41598-025-90318-3
PAX3 expression patterns in ocular surface melanocytes
Abstract
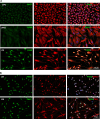
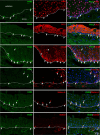
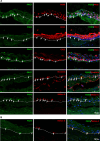
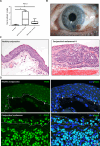
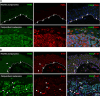
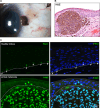
PAX3, a transcription factor essential for neural crest development and melanocyte progenitors, is expressed in various melanocytic tissues. However, its role in ocular surface tissues remains poorly understood. This study investigated the expression patterns of PAX3 in the limbal stem cell niche, specifically in limbal epithelial progenitor cells (LEPC), limbal melanocytes (LM), and limbal mesenchymal stem cells (LMSC). Additionally, PAX3 expression was studied in conjunctival/limbal melanoma specimens. Immunohistochemical analysis revealed predominant PAX3 expression in LM as well in the conjunctival melanocytes, suggesting distinct roles in stem cell regulation and melanocyte maintenance. Notably, PAX3 was significantly upregulated in conjunctival/limbal melanoma tissues compared to healthy counterparts, with expression co-localizing with melanocyte markers (Melan-A, HMB45, SOX10) and the proliferation marker Ki-67 in melanoma cells. These findings suggests that while PAX3 expression is restricted to melanocytes in limbal/conjunctival tissues and its dysregulation may play a crucial role in conjunctival/limbal melanoma development. Further investigation into mechanisms by which PAX3 influences corneal pathophysiology and contributes to conjunctival/limbal melanoma pathogenesis could identify potential therapeutic targets for this aggressive ocular malignancy.
Keywords: Aniridia; Conjunctival melanoma; Limbal epithelial progenitor cells; Limbal melanoma; Limbal niche cells; Limbal stem cell deficiency; Limbal stem cell niche; Limbal stem cells; Melanocytes; Melanoma; Mesenchymal stromal stem cells; PAX3; PAX6.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The Institutional Review Board of the Medical Faculty of the University of Freiburg (25/20) approved experiments. Informed consent: Informed consent to corneal donation had been obtained from the donors or their relatives. Generative AI in scientific writing: During the preparation of this work the authors used Claude AI in order to improve readability and language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Figures


References
-
- Ordonez, P. & Di Girolamo, N. Limbal epithelial stem cells: Role of the Niche microenvironment. Stem Cells. 30, 100–107. 10.1002/stem.794 (2012). - PubMed
-
- Higa, K., Shimmura, S., Miyashita, H., Shimazaki, J. & Tsubota, K. Melanocytes in the corneal Limbus interact with K19-Positive basal epithelial cells. Exp. Eye Res.81, 218–223. 10.1016/j.exer.2005.01.023 (2005). - PubMed
-
- Henkind, P. Migration of limbal melanocytes. Nature214, 1349–1351. 10.1038/2141349b0 (1967). - PubMed
-
- Shimmura, S. & Kawakita, T. Accessory cell populations in the Cornea. Ocul. Surf.4, 74–80. 10.1016/S1542-0124(12)70029-0 (2006). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

