The Conundrum of Treating de novo metastatic Hormone-Sensitive Prostate Cancer
- PMID: 40216859
- PMCID: PMC11992181
- DOI: 10.1038/s41598-025-96065-9
The Conundrum of Treating de novo metastatic Hormone-Sensitive Prostate Cancer
Abstract
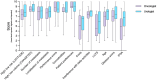
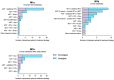
With the heterogeneous use and interpretation of next-generation molecular imaging and approval of new treatment strategies, therapeutic decision-making for de novo metastatic hormone-sensitive prostate cancer (mHSPC) is becoming increasingly challenging. It is conceivable that patients are treated differently in another country, hospital or by another clinician. Here, we aim to provide insights into the clinical practices, challenges, and unmet needs in the management of de novo mHSPC.In this explorative mixed-method study, a survey was sent to urologists and oncologists in 13 Dutch hospitals from the TripleAiM1 network. Additionally, four patient cases were discussed in multi-disciplinary team consultations in four of these hospitals. Results from the survey and patient cases were then discussed in focus group sessions. Three sessions were held with the same expert panel, comprising urologists, medical oncologists, a nuclear medicine physician and radiation oncologist. Major themes were identified and analysed using the Matrix method. Of the 91 surveys distributed, 27 urologists and 19 oncologists responded. Patients with low-volume (LV) disease showed most practice variation; ranging from curative to palliative intent and from single to triplet therapies. Reasons given for this variation include the heterogeneous aspect of LV disease, ambiguous definitions, varying interpretations of study data, lead-time in adoption of novel treatment strategies, and guideline gaps. Adding to this divergence are differences in interpretation of metastatic volume. As the majority of physicians (36/46) use PSMA-PET/CT for staging, while LV and high-volume per CHAARTED criteria are defined on conventional imaging. On a scale of 0-10, metastatic volume (8.5), performance score (8.6), and patient preferences (9.0) were considered the most important factors for selecting treatments. This did not differ significantly between specialties, but showed large dispersion within specialties, suggesting variation at the individual physician level. In conclusion, this study provides insights into clinical practices and challenges in the management of de novo mHSPC. By elucidating the perspectives of Dutch physicians, our findings contribute to a better understanding of the complexities involved in treatment decision-making. Moving forward, there is a need for consensus on definitions, imaging modalities for staging, and treatment selection given the altered diagnostic and therapeutic landscape.
Keywords: Guidelines; Imaging; Metastatic hormone-sensitive prostate cancer; Practice variation; Treatments.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TE reports funding for research from Johnson & Johnson. NM reports grants, institutional and personal fees from Johnson & Johnson. Outside of the submitted work, NM reports grants and personal fees from MSD, AstraZeneca, Astellas, BMS, Pfizer and Bayer. DY reports Astellas consultancy fee. Other authors did not report any relevant conflicts of interests. Ethics approval and consent to participate: In accordance with Dutch legislation, studies of this nature are exempt from the review of a research ethics committee as the participants (i.e., the physicians) were not subjected to procedures or required to follow rules of behaviour. Therefore, this study was not submitted to an ethics committee/ institutional review board for approval. After providing the physicians with adequate information, we considered their consent to complete the survey and participate in the focus groups as sufficient informed consent for the study. The physicians participating in the focus groups explicitly consented to the videotaping of the focus group proceedings. Consent for publication: All participants consented for publication.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

