Rescue of lysosomal acid lipase deficiency in mice by rAAV8 liver gene transfer
- PMID: 40216942
- PMCID: PMC11992068
- DOI: 10.1038/s43856-025-00816-8
Rescue of lysosomal acid lipase deficiency in mice by rAAV8 liver gene transfer
Abstract
Background: Lysosomal acid lipase deficiency (LAL-D) is an autosomal recessive disorder caused by mutations in the LIPA gene, which results in lipid accumulation leading to multi-organ failure. If left untreated, the severe form of LAL-D results in premature death within the first year of life due to failure to thrive and hepatic insufficiency. Weekly systemic injections of recombinant LAL protein, referred as enzyme replacement therapy, is the only available supportive treatment.
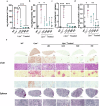
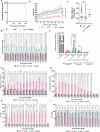
Method: Here, we characterized a novel Lipa-/- mouse model and developed a curative gene therapy treatment based on the in vivo administration of recombinant (r)AAV8 vector encoding the human LIPA transgene under the control of a hepatocyte-specific promoter.
Results: Here we define the minimal rAAV8 dose required to rescue disease lethality and to correct cholesterol and triglyceride accumulation in multiple organs and blood. Finally, using liver transcriptomic and biochemical analysis, we show mitochondrial impairment in Lipa-/- mice and its recovery by gene therapy.
Conclusions: Overall, our in vivo gene therapy strategy achieves a stable long-term LAL expression sufficient to correct the disease phenotype in the Lipa-/- mouse model and offers a new therapeutic option for LAL-D patients.
Plain language summary
Lysosomal acid lipase (LAL) deficiency is a rare genetic disorder that causes toxic lipid build-up in liver and other organs and causes death during childhood if untreated. This study aimed to develop a treatment to cure this disorder by introducing a healthy copy of the LAL gene (gene that causes the disorder). We tested this in a mouse model that lacks this gene and has symptoms similar to the human disorder. We found that this treatment could effectively prevent disease symptoms and reverse damage in affected tissues. This promising gene therapy could offer a potential long-term cure for LAL deficiency, providing hope for patients with this life-threatening condition in the future.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Brown, W. V., Desnick, R. J. & Grabowski, G. A. JCL Roundtable: enzyme replacement therapy for lipid storage disorders. J. Clin. Lipidol.8, 463–472 (2014). - PubMed
-
- Balwani, M. et al. Lysosomal acid lipase deficiency manifestations in children and adults: Baseline data from an international registry. Liver Int. n/a (2023). - PubMed
-
- Bernstein, D. L. et al. Lysosomal acid lipase deficiency allograft recurrence and liver failure- clinical outcomes of 18 liver transplantation patients. Mol. Genet. Metab.124, 11–19 (2018). - PubMed
LinkOut - more resources
Full Text Sources

