Protein disulfide isomerase-enriched extracellular vesicles from bladder cancer cells support tumor survival and malignant transformation in the bladder
- PMID: 40216967
- PMCID: PMC12183083
- DOI: 10.1038/s41388-025-03380-6
Protein disulfide isomerase-enriched extracellular vesicles from bladder cancer cells support tumor survival and malignant transformation in the bladder
Abstract
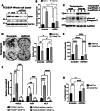
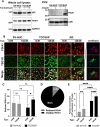
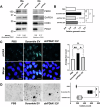
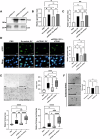
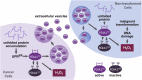
Bladder cancer (BC) patients face high rates of disease recurrence, partially driven by the cancer field effect. This effect is mediated in part by the release of pro-tumorigenic cargos in membrane-enclosed extracellular vesicles (EVs), but the specific underlying mechanisms remain poorly understood. Protein disulfide isomerase (PDIA1) catalyze disulfide bond formation and can help mitigate endoplasmic reticulum (ER) stress, potentially supporting tumor survival. Here, BC cells were found to exhibit better survival under ER stress when PDIA1 was downregulated. These cells maintained homeostatic PDIA1 levels through the EV-mediated release of PDIA1. Chronic exposure of urothelial cells to these PDIA1-enriched BCEVs induced oxidative stress and DNA damage, ultimately leading to the malignant transformation of recipient cells. The EV-transformed cells exhibited DNA damage patterns potentially attributable to oxidative damage, and PDIA1 was found to be a key tumorigenic cargo within EVs. Tissue microarray analyses of BC recurrence confirmed a significant correlation between tumor recurrence and the levels of both PDIA1 and ER stress. Together, these data suggest that cancer cells selectively sort oxidized PDIA1 into EVs for removal, and these EVs can, in turn, induce oxidative stress in recipient urothelial cells, predisposing them to malignant transformation and thereby increasing the risk of recurrence.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. Approval was obtained from the Research Subjects Review Board, the University of Rochester’s Institutional Review Board under STUDY00005593 prior to the construction and use of the Tissue Microarray. Informed consent was obtained from the subjects prior to the tissue collection. All specimens were de-identified to use and no patient identifiers are included in the publication.
Figures

Similar articles
-
Redox signaling-mediated S-glutathionylation of protein disulfide isomerase A1 initiates intrinsic apoptosis and contributes to accelerated aging.Redox Biol. 2025 Sep;85:103680. doi: 10.1016/j.redox.2025.103680. Epub 2025 May 27. Redox Biol. 2025. PMID: 40472774 Free PMC article.
-
Arsenic enhances endoplasmic reticulum stress via YTHDC1/AKR1C3 aix to promote the malignant transformation of human urothelial cells.Toxicol Lett. 2025 Jul;410:199-210. doi: 10.1016/j.toxlet.2025.06.015. Epub 2025 Jun 24. Toxicol Lett. 2025. PMID: 40571110
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Protein disulfide isomerase A1 regulates breast cancer cell immunorecognition in a manner dependent on redox state.Oncol Rep. 2020 Dec;44(6):2406-2418. doi: 10.3892/or.2020.7816. Epub 2020 Oct 20. Oncol Rep. 2020. PMID: 33125139 Free PMC article.
References
-
- Messing EM, Madeb R, Young T, Gilchrist KW, Bram L, Greenberg EB, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107:2173–9. - PubMed
-
- Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. - PubMed
-
- Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomedicine Pharmacother = Biomedecine Pharmacotherapie. 2007;61:299–305. - PubMed
-
- Czerniak B, Dinney C, McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149–74. - PubMed
-
- Jones TD, Wang M, Eble JN, MacLennan GT, Lopez-Beltran A, Zhang S, et al. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin Cancer Res. 2005;11:6512–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

