Mechanism of dracorhodin in accelerating diabetic foot ulcer healing via the Nrf2 pathway, a network pharmacology, molecular docking and experimental validation
- PMID: 40216975
- PMCID: PMC11992152
- DOI: 10.1038/s41598-025-97831-5
Mechanism of dracorhodin in accelerating diabetic foot ulcer healing via the Nrf2 pathway, a network pharmacology, molecular docking and experimental validation
Abstract
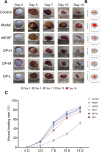
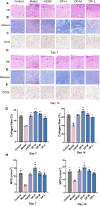
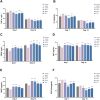
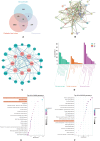
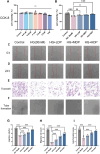
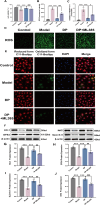
Delayed wound healing in diabetic foot ulcer (DFU) is a major cause of amputations, with ferroptosis impeding recovery. Dracorhodin (DP), a flavonoid from Dragon's Blood, has shown anti-inflammatory and wound-healing properties, though its molecular mechanisms is unclear. This study investigates DP's role in DFU treatment through bioinformatics and experimental approaches. A rat model of DFU was created with a high-fat/high-glucose diet and streptozotocin (STZ) induction, and wound healing was monitored after applying varying DP doses. Histopathological analysis and ELISA assessed tissue changes, inflammatory markers, and growth factors. Network pharmacology and molecular docking were used to identify core targets and pathways, while human umbilical vein endothelial cells (HUVECs) were used for in vitro testing. The results demonstrated that DP accelerated wound healing in DFU rats in a dose-dependent manner by enhancing collagen synthesis, angiogenesis, and growth factor levels, while simultaneously reducing inflammation and ROS levels. Network pharmacology and molecular docking analyses identified the Nrf2-mediated ferroptosis pathway as a potential key mechanism underlying DP's therapeutic effects in DFU. In vitro experiments further revealed that DP improved cell viability and migration, while decreasing ROS and lipid peroxidation levels, effects attributed to Nrf2 pathway activation. These outcomes were significantly attenuated by the Nrf2 inhibitor ML385. In conclusion, DP promotes DFU healing via activation of the Nrf2 pathway and inhibition of ferroptosis.
Keywords: Diabetic foot ulcer; Dracorhodin; Ferroptosis; Nrf2 pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- International Diabetes Federation. The Diabetic Foot. Brussels, Belgium & Federation, I. D. https://www.idf.org/our-activities/care-prevention/diabetic-foot.html (Accessed 1 Aug 2022) (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

