Genetically engineered BMSCs promote dopamine secretion and ameliorate motor dysfunction in a Parkinson's disease rat model
- PMID: 40217082
- PMCID: PMC11992172
- DOI: 10.1038/s41598-025-97557-4
Genetically engineered BMSCs promote dopamine secretion and ameliorate motor dysfunction in a Parkinson's disease rat model
Abstract
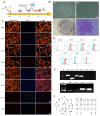
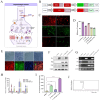
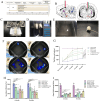
Regenerative therapy based on mesenchymal stem cells (MSCs) is regarded as a promising strategy for treating Parkinson's disease (PD). Previous studies have shown that mesenchymal stem cell transplantation has the potential to treat Parkinson's disease, but its specific mechanism of action is still unclear. In the present study, we generate genetically engineered bone marrow mesenchymal stem cells (BMSCs) encoding three critical genes (TH, DDC, and GCH1) for dopamine synthesis (DA-BMSCs). The DA-BMSCs maintain their MSCs characteristics and stable ability to secrete dopamine after passage. Moreover, the DA-BMSCs survived and functioned in a rat model of PD treated with 6-OHDA 8 weeks after transplantation. Histological studies showed that DA-BMSCs could differentiate into various functional neurons and astrocytes, and DA-BMSCs derived mature dopaminergic neurons extended dense neurites into the host striatum. Importantly, DA-BMSCs promoted the reconstruction of midbrain dopamine pathways by upregulating striatal dopamine and 5-HT levels and downregulating the levels of inflammatory factors including IL-6, TNF-α, and IL-10. These findings suggest that engineered mesenchymal stem cell transplantation for dopamine synthesis may be an attractive donor material for treating Parkinson's disease.
Keywords: Bone marrow mesenchymal stem cells; Dopamine; Dopamine decarboxylase; GTP cyclohydrolase 1; Parkinson’s disease; Tyrosine hydroxylase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All animal experiments were performed in line with the principles of the Chinese Laboratory Animal Management and were approved by the Ethics Committee of Bengbu Medical College (Bengbu, China; approval no. 2022-139). The present study is reported in accordance with ARRIVE guidelines.
Figures

Similar articles
-
[Establishment and characterization of bone marrow mesenchymal stem cell lines stably synthesizing high-level dopamine].Sheng Wu Gong Cheng Xue Bao. 2023 Apr 25;39(4):1773-1788. doi: 10.13345/j.cjb.220718. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 37154338 Chinese.
-
Puerarin promoted proliferation and differentiation of dopamine-producing cells in Parkinson's animal models.Biomed Pharmacother. 2018 Oct;106:1236-1242. doi: 10.1016/j.biopha.2018.07.058. Epub 2018 Jul 20. Biomed Pharmacother. 2018. PMID: 30119192
-
Effects of engineered conserved dopamine neurotrophic factor-expressing bone marrow stromal cells on dopaminergic neurons following 6-OHDA administrations.Mol Med Rep. 2015 Feb;11(2):1207-13. doi: 10.3892/mmr.2014.2878. Epub 2014 Nov 6. Mol Med Rep. 2015. PMID: 25373844
-
Manipulated mesenchymal stem cell therapy in the treatment of Parkinson's disease.Stem Cell Res Ther. 2024 Dec 18;15(1):476. doi: 10.1186/s13287-024-04073-9. Stem Cell Res Ther. 2024. PMID: 39696636 Free PMC article. Review.
-
Catecholamines and Parkinson's disease: tyrosine hydroxylase (TH) over tetrahydrobiopterin (BH4) and GTP cyclohydrolase I (GCH1) to cytokines, neuromelanin, and gene therapy: a historical overview.J Neural Transm (Vienna). 2024 Jun;131(6):617-630. doi: 10.1007/s00702-023-02673-y. Epub 2023 Aug 28. J Neural Transm (Vienna). 2024. PMID: 37638996 Review.
References
-
- Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: A review. J. Am. Med. Assoc.323, 548 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- KJ2021ZD0085/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- KJ2021ZD0085/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- KJ2021ZD0085/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- KJ2021ZD0085/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- KJ2021A0784/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- KJ2021A0774/Natural Science Foundation of the Higher Education Institutions of Anhui Province
- Byycx21050/Postgraduate Innovative Training Program of Bengbu Medical College
- Byycx23006/Postgraduate Innovative Training Program of Bengbu Medical College
- 202310367015/Undergraduate Innovative Training Program of China
- 202310367015/Undergraduate Innovative Training Program of China
- 2021byfy002/Science Research Project of Bengbu Medical College
- 2022e07020032/Anhui Provincial Key Research and Development Project
- 2022e07020030/Anhui Provincial Key Research and Development Project
- 82371382/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

