Remdesivir associated with reduced mortality in hospitalized COVID-19 patients: treatment effectiveness using real-world data and natural language processing
- PMID: 40217145
- PMCID: PMC11992806
- DOI: 10.1186/s12879-025-10817-6
Remdesivir associated with reduced mortality in hospitalized COVID-19 patients: treatment effectiveness using real-world data and natural language processing
Abstract
Background: Remdesivir (RDV) was the first antiviral approved for mild-to-moderate COVID-19 and for those patients at risk for progression to severe disease after clinical trials supported its association with improved outcomes. Real-world evidence (RWE) generated by artificial intelligence techniques could potentially expedite the validation of new treatments in future health crises. We aimed to use natural language processing (NLP) and machine learning (ML) to assess the impact of RDV on COVID19-associated outcomes including time to discharge and in-hospital mortality.
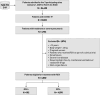
Methods: Using EHRead®, an NLP technology including SNOMED-CT terminology that extracts unstructured clinical information from electronic health records (EHR), we retrospectively examined hospitalized COVID-19 patients with moderate-to-severe pneumonia in three Spanish hospitals between January 2021 and March 2022. Among RDV eligible patients, treated (RDV+) vs untreated (RDV‒) patients were compared after propensity score matching (PSM; 1:3.3 ratio) based on age, sex, Charlson comorbidity index, COVID-19 vaccination status, other COVID-19 treatment, hospital, and variant period. Cox proportional hazards models and Kaplan-Meier plots were used to assess statistical differences between groups.
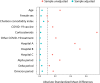
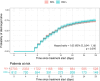
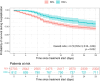
Results: Among 7,651,773 EHRs from 84,408 patients, 6,756 patients were detected with moderate-to-severe COVID-19 pneumonia during the study period. The study population was defined with 4,882 (72.3%) RDV eligible patients. The median age was 72 years and 57.3% were male. A total of 812 (16.6%) patients were classified as RDV+ and were matched to 2,703 RDV‒ patients (from a total of 4,070 RDV‒). After PSM, all covariates had an absolute mean standardized difference of less than 10%. The hazard ratio for in-hospital mortality at 28 days was 0.73 (95% confidence interval, CI, 0.56 to 0.96, p = 0.022) with RDV‒ as the reference group. Risk difference and risk ratio at 28 days was 2.7% and 0.76, respectively, both favoring the RDV+ group. No differences were found in length of hospital stay since RDV eligibility between groups.
Conclusions: Using NLP and ML we were able to generate RWE on the effectiveness of RDV in COVID-19 patients, confirming the potential of using this methodology to measure the effectiveness of treatments in pandemics. Our results show that using RDV in hospitalized patients with moderate-to-severe pneumonia is associated with significantly reduced inpatient mortality. Adherence to clinical guideline recommendations has prognostic implications and emerging technologies in identifying eligible patients for treatment and avoiding missed opportunities during public health crises are needed.
Keywords: Artificial intelligence; COVID-19; Electronic health record; Length of hospital stay; Machine learning; Mortality; Natural language processing; Pneumonia; Propensity score matching; Remdesivir.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was classified as a ‘non-interventional post-authorization study’ by the Spanish Agency of Medicines and Health Products and was reviewed and approved by the Drug Research Ethics Committee of the Balearic Islands (IB 4731/21 EOm) as the reference Ethics Committee. All methods and analysis followed legal and regulatory requirements and generally accepted research practices described in the latest edition of the Declaration of Helsinki, Good Pharmacoepidemiology Practices, and applicable local regulations. Informed consent was waived by the Drug Research Ethics Committee of the Balearic Islands, as data were retrospectively analyzed from patient EHRs, anonymized, and aggregated in an irreversibly dissociated manner. Data collection and assessment were obtained as part of routine clinical activity and performed in a blinded manner. Consent for publication: Not applicable. Competing interests: JRA reports personal fees from Viiv, Janssen, Gilead, MSD, and Aelix outside the submitted work. MPRS, LPH received personal fees from Gilead for patient review. RDMB reports personal fees (speaker fee and congress attendance grants) from Viiv, and Gilead. CDAP and PJBSC are employees of Gilead Sciences. FFL, BDP, PGRP, AFB, and MRJ declare no competing interests.
Figures
References
-
- WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

