Challenges of blinding in clinical balneology trials: a scoping review
- PMID: 40217213
- PMCID: PMC11992841
- DOI: 10.1186/s12906-025-04878-y
Challenges of blinding in clinical balneology trials: a scoping review
Abstract
Background: In evidence-based medicine, randomized, placebo-controlled, double-blind clinical trials are considered the 'gold standard' of study design. Efforts must be made to advance evidence-based balneology in a similar manner. The objective of this scoping review was to assess the intervention types of experimental and control groups used in clinical balneology trials to map the proportion of open-label, single- and double-blind studies.
Methods: Eligibility criteria: i) prospective interventional clinical trial, ii) focused on the therapeutic effect of natural medicinal and mineral water, iii) administered head-out immersion, iv) compared with any other intervention or no treatment, v) in adult patients, and vi) with no restrictions on study design or language. Two authors independently searched the Medline, Embase and Cochrane databases for trials published in any language between 1990 and 12 February 2025.
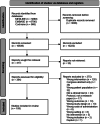
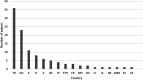
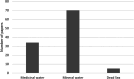
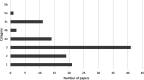
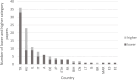
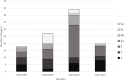
Results: The 109, included trials were categorized into eight groups according to the treatment of the experimental and control groups and the use of blinding. Studies in the lower categories (1, 2, 3) completely lack blinding. In categories 4a-b-c, tap water control was used in parallel with medicinal/mineral water. Category 4c was the first category where the 'gold standard' of evidence-based medicine was implemented. Finally, in the last two categories (5a-b), validated placebo water was used. Low-category papers constituted the largest group, accounting for 74% of the total number of publications. From 1990 to the present, only 11% of publications chose the double-blind setup. Most higher category papers were published in Hungary. Over time, there has been no clear improvement in study design.
Conclusions: Future balneological research should prioritize rigorous experimental designs, particularly by incorporating validated placebo water and double-blind methodologies. Without these improvements, the ability to draw reliable conclusions about the true efficacy of balneotherapy remains limited.
Registration: The scoping review protocol was registered prospectively in OSF registries (Registration DOI https://doi.org/10.17605/OSF . IO/XHS4B, internet Archive link https://archive.org/details/osf-registrations-xhs4b-v1 , Date registered June 26, 2022).
Keywords: Balneology; Blinding; Clinical trial; Medicinal water; Mineral water; Scoping review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Do randomized clinical trials with inadequate blinding report enhanced placebo effects for intervention groups and nocebo effects for placebo groups?Syst Rev. 2014 Feb 21;3:14. doi: 10.1186/2046-4053-3-14. Syst Rev. 2014. PMID: 24555576 Free PMC article.
-
Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 6;22(1):9. doi: 10.1186/s13063-020-04964-1. Trials. 2021. PMID: 33407777 Free PMC article.
-
Head-out immersion in natural thermal mineral water for the management of hypertension: a review of randomized controlled trials.Int J Biometeorol. 2019 Dec;63(12):1707-1718. doi: 10.1007/s00484-019-01780-4. Epub 2019 Aug 11. Int J Biometeorol. 2019. PMID: 31402400 Review.
-
Herbal medicinal products or preparations for neuropathic pain.Cochrane Database Syst Rev. 2019 Apr 2;4(4):CD010528. doi: 10.1002/14651858.CD010528.pub4. Cochrane Database Syst Rev. 2019. PMID: 30938843 Free PMC article.
References
-
- ISO 21426:2018(en). Tourism and related services — Medical spas — Service requirements. https://www.iso.org/obp/ui/fr/#iso:std:iso:21426:ed-1:v1:en .Accessed May 15, 2024.
-
- Government Decree 509/2023. (XI. 20.) on natural healing factors, Annex 2, point 2. https://net.jogtar.hu/jogszabaly?docid=a2300509.kor, Hungarian. Accessed 8 Apr 2025.
-
- MüllerValentini GbR, Mikado GmbH. Bad Kissingen brochure. Entdecke Bad die Zeit. Kissingen, Medicinal water – special guide. Publisher: Bayer. Staatsbad Bad Kissingen GmbH, Germany. https://www.badkissingen.de/ . Accessed May 15, 2024
-
- Balnearios de Espana. Medicinal waters. https://www.balnearios.org/en/medicinal-waters . Accessed May 15, 2024.
-
- People’s Republic of China Ministry of Land. National Standard. Geologic exploration standard of geothermal resources. 2010. https://www.chinesestandard.net/PDF/English.aspx/GBT11615-2010. Accessed May 15, 2024.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

