Neural network analysis as a novel skin outcome in a trial of belumosudil in patients with systemic sclerosis
- PMID: 40217251
- PMCID: PMC11987334
- DOI: 10.1186/s13075-025-03508-9
Neural network analysis as a novel skin outcome in a trial of belumosudil in patients with systemic sclerosis
Abstract
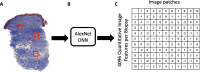
Background: The modified Rodnan skin score (mRSS), a measure of systemic sclerosis (SSc) skin thickness, is agnostic to inflammation and vasculopathy. Previously, we demonstrated the potential of neural network-based digital pathology applied to SSc skin biopsies as a quantitative outcome. Here, we leverage deep learning and histologic analyses of clinical trial biopsies to decipher SSc skin features 'seen' by artificial intelligence (AI).
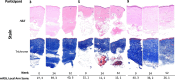
Methods: Adults with diffuse cutaneous SSc ≤ 6 years were enrolled in an open-label trial of belumosudil [a Rho-associated coiled-coil containing protein kinase 2 (ROCK2) inhibitor]. Participants underwent serial mRSS and arm biopsies at week (W) 0, 24 and 52. Two blinded dermatopathologists scored stained sections (e.g., Masson's trichrome, hematoxylin and eosin, CD3, α-smooth muscle actin) for 16 published SSc dermal pathological parameters. We applied our deep learning model to generate QIF signatures/biopsy and obtain 'Fibrosis Scores'. Associations between Fibrosis Score and mRSS (Spearman correlation), and between Fibrosis Score and mRSS versus histologic parameters [odds ratios (OR)], were determined.
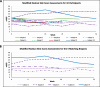
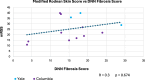
Results: Only ten patients were enrolled due to early study termination, and of those, five had available biopsies due to fixation issues. Median, interquartile range (IQR) for mRSS change (0-52 W) for the ten participants was -2 (-9-7.5) and for the five with biopsies was -2.5 (-11-7.5). The correlation between Fibrosis Score and mRSS was R = 0.3; p = 0.674. Per 1-unit mRSS change (0-52 W), histologic parameters with the greatest associated changes were (OR, 95% CI, p-value): telangiectasia (2.01, [(1.31-3.07], 0.001), perivascular CD3 + (0.99, [0.97-1.02], 0.015), and % of CD8 + among CD3 + (0.95, [0.89-1.01], 0.031). Likewise, per 1-unit Fibrosis Score change, parameters with greatest changes were (OR, p-value): hyalinized collagen (1.1, [1.04 - 1.16], < 0.001), subcutaneous (SC) fat loss (1.47, [1.19-1.81], < 0.001), thickened intima (1.21, [1.06-1.38], 0.005), and eccrine entrapment (1.14, [1-1.31], 0.046).
Conclusions: Belumosudil was associated with non-clinically meaningful mRSS improvement. The histologic features that significantly correlated with Fibrosis Score changes (e.g., hyalinized collagen, SC fat loss) were distinct from those associated with mRSS changes (e.g., telangiectasia and perivascular CD3 +). These data suggest that AI applied to SSc biopsies may be useful for quantifying pathologic features of SSc beyond skin thickness.
Keywords: AlexNet; Artificial intelligence; Belumosudil; Deep Neural Network; Dermal fibrosis; Modified Rodnan skin score; Outcome measure; Outcomes; Scleroderma; Skin fibrosis; Systemic sclerosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by Kadmon Corporation LLC, a Sanofi company, and all participating site’s: Yale University, Columbia University, Northwestern University, and University of California, Los Angeles, Institutional Review Boards. Research partners provided written informed consent in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: MH has received consultancy fees from AbbVie and has received research grant support from Boehringer Ingelheim for an investigator-initiated research project. She is a Scientific Advisory Board Member for the Scleroderma Foundation. She has participated in clinical trials. EJB has received research grants from Kadmon, Boehringer Ingelheim and aTyr. EV has received consulting fees from Boehringer Ingelheim, GSK, Abbvie, BMS, and GLG, has received support from Boehringer Ingelheim, Horizon, Prometheus, Kadmon, and GSK, and serves on the advisory board for Cabaletta and Boehringer Ingelheim. FPW reports research support from AHRQ (R01HS027626), Amgen, and Whoop. FPW reports consulting for Hekaheart, Aura Care, and WndrHlth.
Figures

Update of
-
Neural network analysis as a novel skin outcome in a trial of belumosudil in patients with systemic sclerosis.Res Sq [Preprint]. 2024 Oct 15:rs.3.rs-4889334. doi: 10.21203/rs.3.rs-4889334/v1. Res Sq. 2024. Update in: Arthritis Res Ther. 2025 Apr 11;27(1):85. doi: 10.1186/s13075-025-03508-9. PMID: 39483897 Free PMC article. Updated. Preprint.
References
-
- Hughes M, et al. MRI Digital Artery Volume Index (DAVIX) as a surrogate outcome measure of digital ulcer disease in patients with systemic sclerosis: a prospective cohort study. Lancet Rheumatol. 2023;5(10):e611–21. - PubMed
-
- LeRoy EC, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5. - PubMed
MeSH terms
Substances
Grants and funding
- NIH NICHD K12 HD055884, NIAMS K23 AR059763, R21 AR068035, R01 AR073270/Scleroderma Research Foundation
- R01-AG083735/AG/NIA NIH HHS/United States
- K23-AR-075112/HL/NHLBI NIH HHS/United States
- R01 AG083735/AG/NIA NIH HHS/United States
- K12 HD055884/HD/NICHD NIH HHS/United States
- K23 AR075112/AR/NIAMS NIH HHS/United States
- K23 AR059763/AR/NIAMS NIH HHS/United States
- R01 HL164758/HL/NHLBI NIH HHS/United States
- R01-HL159620/NATIONAL HEART, LUNG, & BLOOD INSTITUTE
- R01 GM141309/GM/NIGMS NIH HHS/United States
- R21 AR068035/AR/NIAMS NIH HHS/United States
- R01 AR073270/AR/NIAMS NIH HHS/United States
- 1R01GM141309/GM/NIGMS NIH HHS/United States
- GR112287/Kadmon Corporation LLC, a Sanofi company
- R01 HL159620/HL/NHLBI NIH HHS/United States
- W81XWH-22-1-0163/Department of Defense grant
- R01 AG062109/AG/NIA NIH HHS/United States
- R01-HL-164758/NATIONAL HEART, LUNG, & BLOOD INSTITUTE
LinkOut - more resources
Full Text Sources
Medical
Research Materials

