The dual roles of chemokines in peripheral nerve injury and repair
- PMID: 40217284
- PMCID: PMC11987372
- DOI: 10.1186/s41232-025-00375-4
The dual roles of chemokines in peripheral nerve injury and repair
Abstract
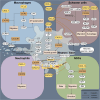
Peripheral nerve injuries (PNI) occur in approximately 13-23 per 100,000 individuals, predominantly affecting young and middle-aged adults. These injuries often require a lengthy recovery period, placing substantial burdens on healthcare systems and national economies. Current treatment strategies have not significantly shortened this lengthy regenerative process, highlighting the urgent need for innovative therapeutic interventions. Chemokines were originally noted for their powerful ability to recruit immune cells; however, as research has advanced, it has become increasingly evident that their role in peripheral nerve repair has been underestimated. In this review, we provide the first comprehensive overview of chemokine expression and activity during peripheral nerve injury and regeneration. We summarize the existing literature on chemokine family members, detailing their expression patterns and localization in injured nerves to facilitate further mechanistic investigations. For chemokines that remain controversial, such as CXCL1 and CCL2, we critically examine experimental methodologies and discuss factors underlying conflicting results, ultimately affirming their contributions to promoting nerve repair. Importantly, we highlight the dual nature of chemokines: in the early stages of injury, they initiate reparative responses, activate Schwann cells, regulate Wallerian degeneration, and support nerve recovery; but when the axons are connected and the repair enters the later stages, their persistent proinflammatory effects during later stages may impede the healing process. Additionally, we emphasize that certain chemokines, including CXCL5, CXCL12, and CCL2, can act directly on neurons/axons, thereby accelerating axonal regeneration. Future research should focus on precisely mapping the localization and temporal expression profiles of these chemokines and exploring therapeutic approaches.
Keywords: Chemokines; Inflammation; Peripheral nerve injuries.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration.J Neuroinflammation. 2022 Jul 12;19(1):179. doi: 10.1186/s12974-022-02497-9. J Neuroinflammation. 2022. PMID: 35820932 Free PMC article.
-
Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury.Acta Neuropathol. 2015 Nov;130(5):605-18. doi: 10.1007/s00401-015-1482-4. Epub 2015 Sep 29. Acta Neuropathol. 2015. PMID: 26419777 Review.
-
Interaction between Schwann cells and other cells during repair of peripheral nerve injury.Neural Regen Res. 2021 Jan;16(1):93-98. doi: 10.4103/1673-5374.286956. Neural Regen Res. 2021. PMID: 32788452 Free PMC article.
-
Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury.J Neuroinflammation. 2011 Aug 30;8:110. doi: 10.1186/1742-2094-8-110. J Neuroinflammation. 2011. PMID: 21878126 Free PMC article. Review.
-
Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury.J Neurosci. 2015 Dec 16;35(50):16431-42. doi: 10.1523/JNEUROSCI.2119-15.2015. J Neurosci. 2015. PMID: 26674868 Free PMC article.
References
-
- Babaei-Ghazani A, Eftekharsadat B, Samadirad B, Mamaghany V, Abdollahian S. Traumatic lower extremity and lumbosacral peripheral nerve injuries in adults: electrodiagnostic studies and patients symptoms. J Forensic Leg Med. 2017;52:89–92. - PubMed
-
- Al-Temimi MH, Chandrasekaran B, Phelan MJ, Pigazzi A, Mills SD, Stamos MJ, et al. Incidence, risk factors, and trends of motor peripheral nerve injury after colorectal surgery: analysis of the national surgical quality improvement program database. Dis Colon Rectum. 2017;60:318–25. - PubMed
-
- Balog BM, Askew T, Lin DL, Kuang M, Hanzlicek B, Damaser MS. The pudendal nerve motor branch regenerates via a brain derived neurotrophic factor mediated mechanism. Exp Neurol. 2020;334: 113438. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

