Matrix metalloproteinase-driven epithelial-mesenchymal transition: implications in health and disease
- PMID: 40217300
- PMCID: PMC11992850
- DOI: 10.1186/s12967-025-06447-w
Matrix metalloproteinase-driven epithelial-mesenchymal transition: implications in health and disease
Abstract
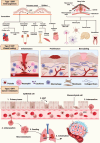
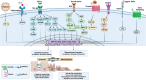
Epithelial-mesenchymal transition (EMT) is a process in which epithelial cells, defined by apical-basal polarity and tight intercellular junctions, acquire migratory and invasive properties characteristic of mesenchymal cells. Under normal conditions, EMT directs essential morphogenetic events in embryogenesis and supports tissue repair. When dysregulated, EMT contributes to pathological processes such as organ fibrosis, chronic inflammation, and cancer progression and metastasis. Matrix metalloproteinases (MMPs)-a family of zinc-dependent proteases that degrade structural components of the extracellular matrix-sit at the nexus of this transition by dismantling basement membranes, activating pro-EMT signaling pathways, and cleaving adhesion molecules. When normally regulated, MMPs promote balanced ECM turnover and support the cyclical remodeling necessary for proper development, wound healing, and tissue homeostasis. When abnormally regulated, MMPs drive excessive ECM turnover, thereby promoting EMT-related pathologies, including tumor progression and fibrotic disease. This review provides an integrated overview of the molecular mechanisms by which MMPs both initiate and sustain EMT under physiological and disease conditions. It discusses how MMPs can potentiate EMT through TGF-β and Wnt/β-catenin signaling, disrupt cell-cell junction proteins, and potentiate the action of hypoxia-inducible factors in the tumor microenvironment. It discusses how these pathologic processes remodel tissues during fibrosis, and fuel cancer cell invasion, metastasis, and resistance to therapy. Finally, the review explores emerging therapeutic strategies that selectively target MMPs and EMT, ranging from CRISPR/Cas-mediated interventions to engineered tissue inhibitors of metalloproteinases (TIMPs), and demonstrates how such approaches may suppress pathological EMT without compromising its indispensable roles in normal biology.
Keywords: Cancer metastasis; Epithelial–mesenchymal transition (EMT); Extracellular matrix (ECM) remodeling; Matrix metalloproteinases (MMPs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium.Int J Mol Sci. 2025 Jun 10;26(12):5553. doi: 10.3390/ijms26125553. Int J Mol Sci. 2025. PMID: 40565017 Free PMC article. Review.
-
Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis.Biomed Pharmacother. 2023 May;161:114472. doi: 10.1016/j.biopha.2023.114472. Epub 2023 Mar 2. Biomed Pharmacother. 2023. PMID: 37002573 Review.
-
Da-yuan-yin decoction alleviates bleomycin-induced pulmonary injury by inhibiting epithelial-mesenchymal transition via E-cadherin/β-catenin complex restoration.J Ethnopharmacol. 2025 Jul 24;351:120148. doi: 10.1016/j.jep.2025.120148. Epub 2025 Jun 14. J Ethnopharmacol. 2025. PMID: 40523450
-
Chondroitin sulfate proteoglycan 4 increases invasion of recessive dystrophic epidermolysis bullosa-associated cutaneous squamous cell carcinoma by modifying transforming growth factor-β signalling.Br J Dermatol. 2024 Dec 23;192(1):104-117. doi: 10.1093/bjd/ljae295. Br J Dermatol. 2024. PMID: 39018437 Free PMC article.
-
The Role of Twisted Gastrulation 1 (TWSG1) Gene in TGF-β Signaling Linked to Cancer: A Comprehensive Review.Asian Pac J Cancer Prev. 2025 Apr 1;26(4):1129-1138. doi: 10.31557/APJCP.2025.26.4.1129. Asian Pac J Cancer Prev. 2025. PMID: 40302064 Free PMC article. Review.
Cited by
-
Inhibitory Impact of the Amino Benzoic Derivative DAB-2-28 on the Process of Epithelial-Mesenchymal Transition in Human Breast Cancer Cells.Molecules. 2025 Aug 5;30(15):3284. doi: 10.3390/molecules30153284. Molecules. 2025. PMID: 40807456 Free PMC article.
-
Advanced nanotherapies for precision treatment of inflammatory lung diseases.Bioact Mater. 2025 Jul 20;53:329-365. doi: 10.1016/j.bioactmat.2025.07.028. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40727485 Free PMC article. Review.
-
Esculetin Attenuates the Migration and Invasion of Human Hepatocellular Carcinoma Cells by Attenuating Matrix Metalloproteinase Activity and Strengthening Tight Junctions.J Cancer Prev. 2025 Jun 30;30(2):111-117. doi: 10.15430/JCP.25.014. J Cancer Prev. 2025. PMID: 40621158 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

