Tissue factor, factor VIII and IX in microvesicle-induced thrombosis and tumor growth of pancreatic cancer
- PMID: 40217494
- PMCID: PMC11987238
- DOI: 10.1186/s12959-025-00715-x
Tissue factor, factor VIII and IX in microvesicle-induced thrombosis and tumor growth of pancreatic cancer
Abstract
Background: Tissue factor (TF)-rich cancer microvesicles are correlated with thrombosis risk. Intrinsic coagulation factors are also associated with the risk of thrombosis in cancer patients. This study explored the roles of pancreatic cancer-derived microvesicles and intrinsic factors in thrombogenesis.
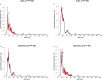
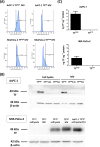
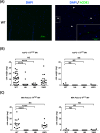
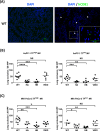
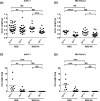
Methods: Human pancreatic cancer cell lines rich in TF (AsPC-1-TFhigh, MIAPaCa-2-TFhigh) or poor in TF [AsPC-1-TFKO(knockout) and MIAPaCa-2-TFlow] were generated for microvesicle preparation and injected into coagulation-defective mice. Inferior vena cava (IVC) clots and lung thrombosis were evaluated. Immunodeficient hemophilia A (NSG-HA) mice were orthotopically injected with the cells mentioned above, and the tumor and IVC clot weights were analyzed.
Results: With the injection of TFhigh microvesicles, IVC clots were rarely found in hemophilic mice. The TFlow and TFKO microvesicles resulted in few IVC clots in any mouse. Lung thrombosis was substantially reduced in the hemophilic mice infused with any microvesicle type. In orthotopic tumor models, TFhigh cells grew faster than did TFlow cells. TFhigh tumor-bearing NSG-WT mice had the most enormous IVC clots, whereas NSG-HA mice had no IVC clots.
Conclusion: Pancreatic cancer thrombosis induced by TF-expressing microvesicles strongly depended on FVIII and FIX, while VWF played a minor role. Moreover, TF, but not FVIII, was significantly related to tumor growth.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. - PubMed
-
- Mulder FI, Horvath-Puho E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, Ay C, Buller HR, Sorensen HT. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137:1959–69. - PubMed
-
- Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, Moik F, Lee AYY. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

