A novel approach utilizing spirocyclic thiopyrimidinone compounds against herpes simplex virus with underlying antiviral mechanisms of action
- PMID: 40217544
- PMCID: PMC11987438
- DOI: 10.1186/s12985-025-02707-9
A novel approach utilizing spirocyclic thiopyrimidinone compounds against herpes simplex virus with underlying antiviral mechanisms of action
Abstract
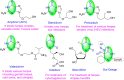
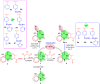
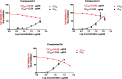
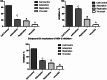
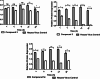
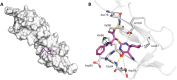
In recent decades, viral outbreaks have significantly threatened global health, with herpes simplex virus type 2 (HSV-2) being one of the most prevalent infections. This study evaluated novel spiropyrimidine derivatives as potential antiviral agents against HSV-2, building on previous research that examined spirocyclic thiopyrimidinone derivatives against human coronavirus 229E (hCoV-229E). Among the eleven synthesized compounds, spiropyrimidinone derivative 3 demonstrated promising antiviral activity, with a selectivity index of 11.2. The drug mechanism of infection studies indicated that compound 3 primarily inhibits HSV-2 at the viral adsorption stage, achieving approximately 83% inhibition and reducing viral multiplication by 34%. Its efficacy is linked to its diketone moiety, which is known for its ability to enhance antiviral effects. Furthermore, the effect of compound 3 on viral inhibition is reflected in the level of caspase-3 protein expression, revealing that the apoptotic pathway is modulated. Docking studies revealed multiple interactions with herpes virus entry mediator (HVEM), indicating its potential as an entry inhibitor. These findings confirm that compound 3 could be a potential candidate for further development in HSV-2 antiviral therapy.
Keywords: Antiviral activity; Herpes simplex virus; Mechanism of action; Spirocyclic thiopyrimidinones.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: We hereby grant our consent for the publication of our manuscript and confirm that all coauthors have agreed to this submission. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Inhibition of herpes simplex type 1 and type 2 infections by Oximacro(®), a cranberry extract with a high content of A-type proanthocyanidins (PACs-A).Antiviral Res. 2016 Aug;132:154-64. doi: 10.1016/j.antiviral.2016.06.006. Epub 2016 Jun 16. Antiviral Res. 2016. PMID: 27321663
-
Early Steps in Herpes Simplex Virus Infection Blocked by a Proteasome Inhibitor.mBio. 2019 May 14;10(3):e00732-19. doi: 10.1128/mBio.00732-19. mBio. 2019. PMID: 31088925 Free PMC article.
-
Antiviral Effects of ABMA against Herpes Simplex Virus Type 2 In Vitro and In Vivo.Viruses. 2018 Mar 9;10(3):119. doi: 10.3390/v10030119. Viruses. 2018. PMID: 29522484 Free PMC article.
-
Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers.Antimicrob Agents Chemother. 2011 Jul;55(7):3231-9. doi: 10.1128/AAC.00149-11. Epub 2011 May 16. Antimicrob Agents Chemother. 2011. PMID: 21576438 Free PMC article.
-
Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections.Med Res Rev. 2017 Jan;37(1):149-179. doi: 10.1002/med.21405. Epub 2016 Aug 12. Med Res Rev. 2017. PMID: 27518199 Review.
References
-
- Ostlund EN. The equine herpesviruses. Vet Clin North Am Equine Pract. 1993;9(2):283–94. 10.1016/s0749-0739(17)30396-6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

