Ozenoxacin 1% in Pediatric and Adult Patients with Impetigo: A Meta-Analysis of Randomized Trials
- PMID: 40217608
- PMCID: PMC11989652
- DOI: 10.3390/jcm14072157
Ozenoxacin 1% in Pediatric and Adult Patients with Impetigo: A Meta-Analysis of Randomized Trials
Abstract
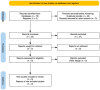
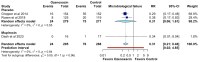
Introduction: Impetigo is a relatively common superficial infection of the skin and soft tissues. Although its prevalence is more significant in childhood, it might also occur in adulthood, affecting the quality of life of our patients. Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) comparing ozenoxacin 1% with placebo or mupirocin was conducted. Databases searched included PubMed, Embase, Cochrane Library, and ClinicalTrials.gov. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were estimated using a random-effects model, and heterogeneity was assessed using I2 statistics. Results: Four RCTs with 754 patients met the inclusion criteria. Ozenoxacin significantly improved the clinical success (RR: 1.14, 95% CI: 1.04-1.26, and I2 = 0%) and reduced the clinical failure (RR: 0.54, 95% CI: 0.39-0.75, and I2 = 0%) compared to the placebo. Microbiological success was also superior (RR: 1.31, 95% CI: 1.05-1.58, and I2 = 4%), while the microbiological failure was significantly lower (RR: 0.31, 95% CI: 0.21-0.46, and I2 = 0%). Comparisons with mupirocin showed similar efficacy, though the estimates were less precise. Conclusions: Ozenoxacin 1% is an effective treatment for impetigo, significantly improving clinical and microbiological outcomes while reducing the failure rates compared to the placebo. Its efficacy is comparable to mupirocin, suggesting it as a viable alternative for first-line therapy. Given the low heterogeneity observed, these findings support the clinical use of ozenoxacin for impetigo management. Future large-scale RCTs and direct comparative studies are warranted to further validate its therapeutic benefits.
Keywords: adolescents; adults; children; impetigo; ozenoxacin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Efficacy and Safety of Ozenoxacin Cream for Treatment of Adult and Pediatric Patients With Impetigo: A Randomized Clinical Trial.JAMA Dermatol. 2018 Jul 1;154(7):806-813. doi: 10.1001/jamadermatol.2018.1103. JAMA Dermatol. 2018. PMID: 29898217 Free PMC article. Clinical Trial.
-
Topical Antibacterial Agent for Treatment of Adult and Pediatric Patients With Impetigo: Pooled Analysis of Phase 3 Clinical Trials.J Drugs Dermatol. 2018 Oct 1;17(10):1051-1057. J Drugs Dermatol. 2018. PMID: 30365584 Clinical Trial.
-
Topical Ozenoxacin Cream 1% for Impetigo: A Review.J Drugs Dermatol. 2019 Jul 1;18(7):655-661. J Drugs Dermatol. 2019. PMID: 31334625 Review.
-
Ozenoxacin 1% cream in the treatment of impetigo: a multicenter, randomized, placebo- and retapamulin-controlled clinical trial.Future Microbiol. 2014;9(9):1013-23. doi: 10.2217/fmb.14.78. Future Microbiol. 2014. PMID: 25340832 Clinical Trial.
-
Ozenoxacin: A Novel Topical Quinolone for Impetigo.Ann Pharmacother. 2018 Dec;52(12):1233-1237. doi: 10.1177/1060028018786510. Epub 2018 Jul 2. Ann Pharmacother. 2018. PMID: 29962213 Review.
References
-
- Bell L., Whyte A., Duysburgh C., Marzorati M., Van den Abbeele P., Le Cozannet R., Fança-Berthon P., Fromentin E., Williams C. A randomized, placebo-controlled trial investigating the acute and chronic benefits of American Ginseng (Cereboost®) on mood and cognition in healthy young adults, including in vitro investigation of gut microbiota changes as a possible mechanism of action. Eur. J. Nutr. 2022;61:413–428. - PMC - PubMed
-
- Flores R., Villarroel J.L., Valenzuela F. Enfrentamiento de las infecciones de piel en el adulto. Rev. Med. Clin. Condes. 2021;32:429–441. doi: 10.1016/j.rmclc.2021.06.004. - DOI
-
- Rosen T., Albareda N., Rosenberg N., Alonso F.G., Roth S., Zsolt I., Hebert A.A. Efficacy and Safety of Ozenoxacin Cream for Treatment of Adult and Pediatric Patients With Impetigo: A Randomized Clinical Trial. JAMA Dermatol. 2018;154:806–813. doi: 10.1001/jamadermatol.2018.1103. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

