Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study
- PMID: 40217623
- PMCID: PMC11989484
- DOI: 10.3390/jcm14072172
Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study
Abstract
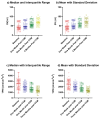
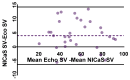
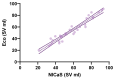
Background/Objectives: Heart failure (HF) with reduced ejection fraction remains a significant global health challenge despite advances in medical therapy. Cardiac contractility modulation (CCM) is a promising treatment for symptomatic HF patients who are ineligible for cardiac resynchronization therapy (CRT). Non-invasive methods to assess the acute hemodynamic effects of CCM are critical to optimize care and guide treatment. This study aimed to evaluate the acute impact of CCM on stroke volume (SV) and total peripheral resistance index (TPRI) using the non-invasive bioimpedance-based system (NICaS®). Methods: Eight HF patients (median age: 64.6 years, median left ejection fraction (LVEF): 34.5%) underwent implantation of the Optimizer Smart Mini CCM device. Hemodynamic parameters, including SV and TPRI, were measured using NICaS® at baseline (pre-implantation) and at 1 week, 1 month, and 3 months post-implantation. Measurements were repeated eight times per session and analyzed using non-parametric statistical tests, including the Kruskal-Wallis test, Mann-Whitney test, and Kolmogorov-Smirnov test. Results: Median SV increased significantly from 40.02 mL (interquartile range (IQR): 32.62-78.16 mL) at baseline to 69.83 mL (IQR: 58.63-86.36 mL) at 3 months (p < 0.0001). Median TPRI decreased significantly from 2537 dn s/cm5 m2 (IQR: 1807-3084 dn s/cm5 m2) to 1307 dn s/cm5 m2 (IQR: 1119-1665 dn s/cm5 m2) over the same period (p < 0.0001). CCM therapy significantly improved SV and reduced TPRI in HF patients within three months of implantation. Conclusions: NICaS® provided a reliable, non-invasive tool for monitoring these acute hemodynamic changes, supporting its use in clinical practice.
Keywords: NICaS®; cardiac contractility modulation; heart failure; hemodynamics; non-invasive monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Continuous non-invasive measurement of stroke volume and cardiac index in infants and children: comparison of Impedance Cardiography NICaS® vs CardioQ® method.Clin Ter. 2018 May-Jun;169(3):e110-e113. doi: 10.7417/T.2018.2064. Clin Ter. 2018. PMID: 29938742
-
New Generation Cardiac Contractility Modulation Device-Filling the Gap in Heart Failure Treatment.J Clin Med. 2019 Apr 29;8(5):588. doi: 10.3390/jcm8050588. J Clin Med. 2019. PMID: 31035648 Free PMC article.
-
Noninvasive Hemodynamic Evaluation Following TAVI for Severe Aortic Stenosis.J Am Heart Assoc. 2023 Apr 4;12(7):e028479. doi: 10.1161/JAHA.122.028479. Epub 2023 Mar 21. J Am Heart Assoc. 2023. PMID: 36942754 Free PMC article.
-
Cardiac contractility modulation in patients with heart failure - A review of the literature.Heart Fail Rev. 2024 May;29(3):689-705. doi: 10.1007/s10741-024-10390-1. Epub 2024 Feb 23. Heart Fail Rev. 2024. PMID: 38393423 Review.
-
Correlation of Impedance Cardiography-Derived and Cardiac Magnetic Resonance-Derived Stroke Volumes.Curr Probl Cardiol. 2023 Feb;48(2):101457. doi: 10.1016/j.cpcardiol.2022.101457. Epub 2022 Oct 21. Curr Probl Cardiol. 2023. PMID: 36273652 Review.
References
-
- Tomasoni D., Pagnesi M., Colombo G., Chiarito M., Stolfo D., Baldetti L., Lombardi C.M., Adamo M., Maggi G., Inciardi R.M., et al. Guideline-directed medical therapy in severe heart failure with reduced ejection fraction: An analysis from the HELP-HF registry. Eur. J. Heart Fail. 2024;26:327–337. doi: 10.1002/ejhf.3081. - DOI - PubMed
-
- Bazoukis G., Saplaouras A., Efthymiou P., Yiannikourides A., Liu T., Efremidis M., Lampropoulos K., Xydonas S., Tse G., Armoundas A.A. Cardiac contractility modulation in patients with heart failure—A review of the literature. Heart Fail. Rev. 2024;29:689–705. doi: 10.1007/s10741-024-10390-1. - DOI - PubMed
-
- Rajagopalan N., Borlaug B.A., Bailey A.L., Eckman P.M., Guglin M., Hall S., Montgomery M., Ramani G., Khazanie P. Practical guidance for hemodynamic assessment by right heart catheterization in management of heart failure. JACC Heart Fail. 2024;12:1141–1156. doi: 10.1016/j.jchf.2024.03.020. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

