Real-World Evidence of Administration of Biologic Agents in Patients with Severe Asthma: An Analysis of the Respiratory Department of University Hospital of Patras Asthma Registry
- PMID: 40217624
- PMCID: PMC11989620
- DOI: 10.3390/jcm14072174
Real-World Evidence of Administration of Biologic Agents in Patients with Severe Asthma: An Analysis of the Respiratory Department of University Hospital of Patras Asthma Registry
Abstract
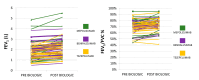
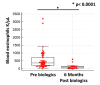
Background: Real-world data on currently used biologic agents in patients with severe asthma are lacking. Methods: In this retrospective study, we recorded between 16 May 2020 and 31 December 2024 consecutive patients who presented to our asthma outpatient clinic received a diagnosis of uncontrolled severe asthma and were treated with biologic agents. Outcomes included a comparison of disease phenotypic characteristics, as well as asthma control, lung function, longitudinal use of corticosteroids, and hospitalizations due to exacerbations at baseline and post-biologic treatment at 6-month follow-up. Results: We identified 80 patients with uncontrolled severe asthma treated with biologic agents. The median age (95% CI) at the time of diagnosis was 67.0 (61.0 to 70.0) years. Most patients were female (65.0%, n = 52) and never smokers (51.3%, n = 41). The median value of ACT (95% CI) was 15 (15 to 16) at the time of diagnosis. The mean FEV1% predicted ±SD at the baseline was 68.9 ± 22.0. The median value of blood eosinophils (95% CI) was 365 (252 to 448) K/μL in the overall population. One-third (36.3%) of patients were hospitalized due to severe asthma exacerbation in the previous year. Longitudinal use of oral corticosteroids was recorded in 11.3% of included patients. Three patients (3.8%) were treated with omalizumab, 23 patients (28.8%) with mepolizumab, 33 patients (41.2%) with benralizumab and 21 patients (26.2%) with tezepelumab. The median value of ACT (95% CI) post-biologic treatment at 6-month follow-up was 20 (20 to 21), p < 0.0001. The mean FEV1% predicted ±SD at 6-month follow-up was 77.6 ± 25.2, p = 0.12. The median value of blood eosinophils (95% CI) 6 months after initiation of biologic treatment was 100 (40 to 121) K/μL, p < 0.0001. Elimination of hospitalizations due to asthma flares was recorded in 97.5% of patients (p < 0.0001). With regard to the longitudinal use of oral corticosteroids, we noticed that 96.2% of patients achieved discontinuation. No treatment-related adverse events were noticed. Conclusions: The administration of current biologic agents in patients with severe asthma seems to be both effective and safe, sparing the toxicity of oral corticosteroids.
Keywords: benralizumab; biologic agents; mepolizumab; severe asthma; tezepelumab.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- FitzGerald J.M., Tran T.N., Alacqua M., Altraja A., Backer V., Bjermer L., Bjornsdottir U., Bourdin A., Brusselle G., Bulathsinhala L., et al. International severe asthma registry (ISAR): Protocol for a global registry. BMC Med. Res. Methodol. 2020;20:212. doi: 10.1186/s12874-020-01065-0. - DOI - PMC - PubMed
-
- Holguin F., Cardet J.C., Chung K.F., Diver S., Ferreira D.S., Fitzpatrick A., Gaga M., Kellermeyer L., Khurana S., Knight S., et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. - DOI - PubMed
LinkOut - more resources
Full Text Sources

