Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis
- PMID: 40217680
- PMCID: PMC11989280
- DOI: 10.3390/jcm14072232
Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis
Abstract
Background/Objectives: Evidence is needed on the real-world outcomes of upadacitinib in patients with ulcerative colitis. This systematic review and meta-analysis evaluated the real-world effectiveness of upadacitinib for active UC. Methods: The primary outcome was clinical remission evaluated at week 8. Secondary outcomes included response, steroid-free remission, biochemical remission, colectomy, and safety. A random-effects meta-analysis model was used to calculate the pooled effect sizes (percentages or incidence rates) of effectiveness and safety outcomes. Results: Twenty-four studies with 1388 patients were included. Ninety-four percent of patients had previously failed biologics or Janus kinase inhibitors (JAKi), including 53.2% with tofacitinib. Clinical remission at week 8 was achieved in 68.4% of patients (95% confidence interval 55.5-80.2). Clinical remission was achieved in 48.3%, 71.1%, and 64.6% of patients at weeks 2 to 6, 12 to 16, and 24 to 36, respectively. Response was achieved in 72.6%, 82.1%, and 78.7% of patients at weeks 2 to 6, week 8, and weeks 12 to 16, respectively. Steroid-free remission was achieved in 39% of patients at week 8. Upadacitinib results were unaffected by prior biologic or JAKi failure. Mean fecal calprotectin level decreased from 1485.0 µ/g at baseline to 454.8 µ/g post-treatment (p < 0.01). The mean CRP level decreased from 12.3 mg/L at baseline to 4.4 mg/L post-treatment (p = 0.02). The incidence rates of colectomy, serious adverse events, and herpes zoster were 13.3, 2.3, and 1.7 per 100 patient-years, respectively. Conclusions: This meta-analysis confirms the effectiveness and safety of upadacitinib in a highly treatment-refractory population of UC patients.
Keywords: Janus kinase; meta-analysis; real-world effectiveness; safety; ulcerative colitis; upadacitinib.
Conflict of interest statement
C.T. has served as a speaker, consultant, and advisory board member for MSD, AbbVie, Pfizer, Takeda, Galapagos, Lilly, Sandoz, Fresenius Kabi, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, and Tillots. These activities were not related to the present work. The remaining authors declare no conflict of interest.
Figures

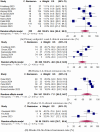


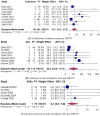

References
-
- Danese S., Vermeire S., Zhou W., Pangan A.L., Siffledeen J., Greenbloom S., Hébuterne X., D’Haens G., Nakase H., Panés J., et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. - PubMed
-
- Panaccione R., Lichtenstein G., Nakase H., Armuzzi A., Kucharzik T., Levy G., Palac H., Kujawski M., Klaff J., Cheon J.H. Safety of upadacitinib in ulcerative colitis: Long-term data from the phase 3 open-label extension study (U-ACTIVATE) Gastroenterology. 2023;164:S-1100.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

