Tracheal Diverticula in People with Cystic Fibrosis on Elexacaftor/Tezacaftor/Ivacaftor: An Italian Multicenter Retrospective Study
- PMID: 40217771
- PMCID: PMC11989805
- DOI: 10.3390/jcm14072320
Tracheal Diverticula in People with Cystic Fibrosis on Elexacaftor/Tezacaftor/Ivacaftor: An Italian Multicenter Retrospective Study
Abstract
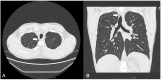
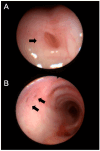
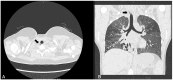
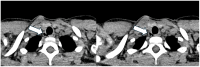
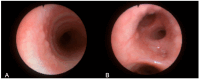
Background/Objectives: Cystic Fibrosis (CF) is an autosomal recessive genetic disorder caused by variants in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Recently, a targeted therapy for CF has been developed, represented by the CFTR modulators that enhance or restore the function of the CFTR protein. The most recent is the combination of three modulators, Elexacaftor, Tezacaftor, and Ivacaftor (ETI). This study describes the presentation, management, and follow-up of tracheal diverticulum (TD) in pwCF receiving ETI therapy. Methods: This retrospective study included people with CF (pwCF) on ETI treatment and followed up in two CF Italian centers who developed an asymptomatic TD, diagnosed incidentally at chest CT scan. Results: Among 268 pwCF receiving ETI, three (1.19%) were diagnosed with TD identified after chest CT and were included in this study. Endoscopic confirmation was obtained in one patient. All patients were on inhaled colistimethate, two of them for chronic Pseudomonas aeruginosa colonization, and one undergoing eradication therapy. Conclusions: TD may be identified in chest CT obtained in pwCF in treatment with ETI. Further studies and a longer follow up are needed to confirm these findings.
Keywords: bronchoscopy; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor; tracheal diverticulum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Regulatory T cell enhancement in adults with cystic fibrosis receiving Elexacaftor/Tezacaftor/Ivacaftor therapy.Front Immunol. 2023 Feb 16;14:1107437. doi: 10.3389/fimmu.2023.1107437. eCollection 2023. Front Immunol. 2023. PMID: 36875141 Free PMC article.
-
Perceived burden of respiratory physiotherapy in people with cystic fibrosis taking elexacaftor-tezacaftor-ivacaftor combination: a 1-year observational study.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241235054. doi: 10.1177/17534666241235054. Ther Adv Respir Dis. 2024. PMID: 38554035 Free PMC article.
-
Eradication of Nontuberculous Mycobacteria in People with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor: A Multicenter Cohort Study.J Cyst Fibros. 2024 Jan;23(1):41-49. doi: 10.1016/j.jcf.2023.05.003. Epub 2023 May 10. J Cyst Fibros. 2024. PMID: 37173154
-
The Effects of Elexacaftor, Tezacaftor, and Ivacaftor (ETI) on Blood Glucose in Patients With Cystic Fibrosis: A Systematic Review.Cureus. 2023 Jul 11;15(7):e41697. doi: 10.7759/cureus.41697. eCollection 2023 Jul. Cureus. 2023. PMID: 37575762 Free PMC article. Review.
References
-
- Sly P.D., Brennan S., Gangell C., de Klerk N., Murray C., Mott L., Stick S.M., Robinson P.J., Robertson C.F., Ranganathan S.C., et al. Lung Disease at Diagnosis in Infants with Cystic Fibrosis Detected by Newborn Screening. Am. J. Respir. Crit. Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. - DOI - PubMed
LinkOut - more resources
Full Text Sources

