Diagnostic and Therapeutic Approaches for Spinal Subarachnoid Hemorrhage Due to Spinal Aneurysms and Other Etiologies
- PMID: 40217848
- PMCID: PMC11989469
- DOI: 10.3390/jcm14072398
Diagnostic and Therapeutic Approaches for Spinal Subarachnoid Hemorrhage Due to Spinal Aneurysms and Other Etiologies
Abstract
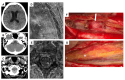
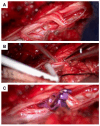
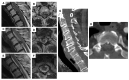
Background: Spinal subarachnoid hemorrhage (sSAH) is a very rare disease. Detailed information about the natural course, pathogenesis, radiological manifestation, and therapeutic management is lacking. This study aimed to analyze patients diagnosed with sSAH, focusing on the origin, management strategies, and therapeutic approaches to sSAH. Methods: The study included a cohort of patients admitted to the Department of Neurosurgery, LMU University Hospital, LMU Munich, between January 2021 and December 2024 with a confirmed diagnosis of spinal subarachnoid hemorrhage and, among other things, spinal aneurysms. Data on the included patients were recorded with emphasis on demographics, radiological examination (CT, MRI, and DSA), aneurysm-specific characteristics, and clinical outcome. Results: The study included six patients diagnosed with spinal subarachnoid hemorrhage via multimodal imaging. The etiology of sSAH was identified in all cases, encompassing spinal aneurysms in three patients, anticoagulation therapy in two cases, and bony microspurs in one case, with management strategies tailored as either conservative (monitoring and imaging) or surgical (aneurysm resection, arterial feeder coagulation, or evacuation of intraspinal bleeding). No major adverse events were observed, and all the patients demonstrated neurological improvement or exhibited only mild-to-moderate disability during follow-up. Conclusions: Spinal subarachnoid hemorrhage can be due to a ruptured spinal aneurysm, but in some cases, other underlying causes should be considered as the source of the hemorrhage. Given the scarcity of literature on this condition, it is crucial to identify the correct diagnosis and implement a patient-tailored therapeutic approach.
Keywords: SAH; clipping; neurosurgery; spinal aneurysm; spinal subarachnoid hemorrhage; subarachnoid hemorrhage; vascular disorder; vascular pathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Ruptured Spinal Aneurysms: Diagnosis and Management Paradigms.World Neurosurg. 2021 Feb;146:e368-e377. doi: 10.1016/j.wneu.2020.10.098. Epub 2020 Oct 23. World Neurosurg. 2021. PMID: 33223127
-
Exploring Spinal Subarachnoid Hemorrhage: A Neurosurgical Case Series.Cureus. 2023 Sep 20;15(9):e45627. doi: 10.7759/cureus.45627. eCollection 2023 Sep. Cureus. 2023. PMID: 37868412 Free PMC article.
-
Spinal subarachnoid hemorrhage and aneurysms.Handb Clin Neurol. 2017;143:215-223. doi: 10.1016/B978-0-444-63640-9.00020-5. Handb Clin Neurol. 2017. PMID: 28552143 Review.
-
Coil embolization for intracranial aneurysms: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(1):1-114. Epub 2006 Jan 1. Ont Health Technol Assess Ser. 2006. PMID: 23074479 Free PMC article.
-
Lateral Spinal Artery Aneurysm Causing Subarachnoid Hemorrhage: Literature Review and Case Report.J Clin Med. 2024 Aug 20;13(16):4910. doi: 10.3390/jcm13164910. J Clin Med. 2024. PMID: 39201057 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

