The Current Landscape of Clinical Trials
- PMID: 40217968
- PMCID: PMC11989625
- DOI: 10.3390/jcm14072519
The Current Landscape of Clinical Trials
Abstract
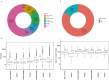
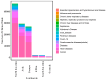
Background/Objectives: Clinical trials are essential in the development of new medical treatments, offering crucial data on their safety and effectiveness. Methods: This study provides a comprehensive analysis of clinical trials registered on ClinicalTrials.gov, examining the current landscape, challenges, and innovations that have shaped the field over the past century. Data were extracted on 7 March 2025 and analyzed to identify patterns in trial design, sponsorship, participant demographics, and geographical distribution. Results: The analysis reveals a continuous increase in clinical trial registrations, peaking in 2021, driven by the COVID-19 pandemic. Most trials focus on cancer, reflecting its global burden, with randomized controlled trials (RCTs) being the most common study design. However, challenges persist, including underrepresentation of certain demographics, limited global distribution, and insufficient reporting of trial results. Additionally, the underrepresentation of pediatric, elderly, and minority populations in trials limits the generalizability of findings. Conclusions: The analysis underscores the need for more inclusive and globally distributed research to address disparities in health outcomes.
Keywords: clinical trial report; clinical trials; clinicaltrials.gov; randomized controlled trials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2020 Nov 20;11(11):CD006207. doi: 10.1002/14651858.CD006207.pub5. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Jan 30;1:CD006207. doi: 10.1002/14651858.CD006207.pub6. PMID: 33215698 Free PMC article. Updated.
-
Factors Associated With Age Disparities Among Cancer Clinical Trial Participants.JAMA Oncol. 2019 Dec 1;5(12):1769-1773. doi: 10.1001/jamaoncol.2019.2055. JAMA Oncol. 2019. PMID: 31158272 Free PMC article.
-
Disparities in positive results and dissemination of randomized controlled trials in immuno-oncology.Int Rev Immunol. 2023;42(2):91-100. doi: 10.1080/08830185.2022.2088744. Epub 2022 Jun 17. Int Rev Immunol. 2023. PMID: 35712868
-
CAR T-cell therapy landscape in pediatric, adolescent and young adult oncology - A comprehensive analysis of clinical trials.Crit Rev Oncol Hematol. 2025 May;209:104648. doi: 10.1016/j.critrevonc.2025.104648. Epub 2025 Feb 1. Crit Rev Oncol Hematol. 2025. PMID: 39900318 Review.
References
-
- Learn About Studies. ClinicalTrials.gov. [(accessed on 14 March 2025)]. Available online: https://clinicaltrials.gov/study-basics/learn-about-studies#q1.
-
- Mills E.J., Wathen J.K., Park J.J.H., editors. Introduction to Adaptive Trial Designs and Master Protocols. Cambridge University Press; Cambridge, UK: 2023. Introduction and History of Clinical Trial Research; pp. 1–20.
-
- Ballman K.V. Introduction to Clinical Trials, Clinical Trial Designs, and Statistical Terminology Used for Predictive Biomarker Research and Validation. In: Badve S., Kumar G.L., editors. Predictive Biomarkers in Oncology: Applications in Precision Medicine. Springer International Publishing; Cham, Switzerland: 2019. pp. 19–36. - DOI
-
- What Are Clinical Trials?—NCI. 2023. [(accessed on 14 March 2025)]. Available online: https://www.cancer.gov/research/participate/clinical-trials/what-are-cli....
LinkOut - more resources
Full Text Sources
Miscellaneous

