Assessment of Inter-Reader Reliability of Fazekas Scoring on Magnetic Resonance Imaging of the Brain in Adult Patients with Sickle Cell Disease
- PMID: 40218207
- PMCID: PMC11988997
- DOI: 10.3390/diagnostics15070857
Assessment of Inter-Reader Reliability of Fazekas Scoring on Magnetic Resonance Imaging of the Brain in Adult Patients with Sickle Cell Disease
Abstract
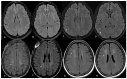
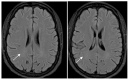
Background/Objectives: Cerebral white matter disease is a common finding in patients with sickle cell that has been linked to cognitive impairment. However, there is no standardized approach for quantification of the cerebral disease burden. The Fazekas score is widely used to quantify the burden of white matter disease in chronic small vessel disease. However, its utility in sickle cell disease, specifically the inter-rater variability, has not been established. Methods: A patient cohort was compiled for the purpose of a research ethics board (REB)-approved retrospective study of adult patients with sickle cell disease, each of whom underwent MRI/MRA between the years 2017 and 2019. A total of 90 such patients were captured. All MRI/MRA studies were performed on three Tesla MRIs. Two independent neuroradiologists assessed the axial FLAIR MRI brain sequence (see image 1) for each of the 90 patients, with the sole focus of assigning a Fazekas score (0-3) to each study as a means of quantifying the burden of ischemic white matter lesions. The neuroradiologists were blinded to the scoring assigned by their counterpart and to the clinical information. After the initial assessment was completed, studies with discrepant Fazekas scores were documented and discussed by both readers. A consensus Fazekas score was then assigned to each of these studies. Results: Cohen's weighted kappa was used as a measure of agreement between readers. The expected agreement was 74.65%, with an observed agreement of 94.44% between readers, with a kappa of 0.7808. Conclusions: We conclude on the basis of our study that there is good inter-reader reliability of Fazekas scoring on axial FLAIR MRI brain sequence in patients with sickle cell disease. The Fazekas is a promising measure that could easily be integrated in systematic evaluation of cerebrovascular lesions of adults with sickle cell disease.
Keywords: Fazekas scoring tool; magnetic resonance imaging; sickle cell disease; white matter disease.
Conflict of interest statement
The authors declare no conflicts of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Figures
Similar articles
-
Can white matter hyperintensities based Fazekas visual assessment scales inform about Alzheimer's disease pathology in the population?Alzheimers Res Ther. 2024 Jul 10;16(1):157. doi: 10.1186/s13195-024-01525-5. Alzheimers Res Ther. 2024. PMID: 38987827 Free PMC article.
-
Tissue Sodium Concentration within White Matter Correlates with the Extent of Small Vessel Disease.Cerebrovasc Dis. 2021;50(3):347-355. doi: 10.1159/000514133. Epub 2021 Mar 17. Cerebrovasc Dis. 2021. PMID: 33730735
-
Can white matter hyperintensities based Fazekas visual assessment scales inform about Alzheimer's disease pathology in the population?Res Sq [Preprint]. 2024 Mar 11:rs.3.rs-4017874. doi: 10.21203/rs.3.rs-4017874/v1. Res Sq. 2024. Update in: Alzheimers Res Ther. 2024 Jul 10;16(1):157. doi: 10.1186/s13195-024-01525-5. PMID: 38558965 Free PMC article. Updated. Preprint.
-
Visual Assessment of Age-Related White Matter Hyperintensities Using FLAIR Images at 3 T: Inter- and Intra-Rater Agreement.Neurodegener Dis. 2016;16(3-4):279-83. doi: 10.1159/000441420. Epub 2015 Dec 9. Neurodegener Dis. 2016. PMID: 26646220
-
Development and Validation of a Fusion Model Based on Carotid Plaques and White Matter Lesion Burden Imaging Characteristics to Evaluate Ischemic Stroke Severity in Symptomatic Patients.J Magn Reson Imaging. 2025 Feb;61(2):648-660. doi: 10.1002/jmri.29439. Epub 2024 May 13. J Magn Reson Imaging. 2025. PMID: 38738856
References
-
- Rampersaud E., Palmer L.E., Hankins J.S., A Sheehan V., Bi W., Mulder H., Kang G., Estepp J.H., Wang S., Thrasher A., et al. Precision Medicine for Sickle Cell Disease through Whole Genome Sequencing. Blood. 2018;132((Suppl. S1)):3641.
-
- CDC Website 2024. [(accessed on 30 December 2024)]; Available online: https://www.cdc.gov/sickle-cell/data/index.html#:~:text=Sickle%20cell%20....
-
- Rees D.C., Brousse V.A., Brewin J.N. Determinants of severity in sickle cell disease. Blood Rev. 2022;56:100983. - PubMed
LinkOut - more resources
Full Text Sources

