Neuroendocrine Tumor Metastases to the Breast Mimic Breast Primary Carcinoma: Mammography and Multimodality US Assessment in Challenging Differential Diagnosis
- PMID: 40218210
- PMCID: PMC11988801
- DOI: 10.3390/diagnostics15070860
Neuroendocrine Tumor Metastases to the Breast Mimic Breast Primary Carcinoma: Mammography and Multimodality US Assessment in Challenging Differential Diagnosis
Abstract
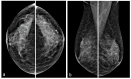
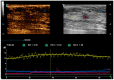
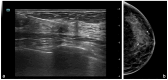
Metastases to the breast from non-mammary malignancies are rare, accounting for 0.1-5% of all breast malignancies. Neuroendocrine tumors (NETs) rarely metastasize to the breast. PET-CT somatostatin receptor imaging plays a pivotal role in the staging and follow-up of NETs, leveraging tracers like 68Ga-DOTATOC that bind to somatostatin receptors (SSTRs) expressed on tumor cells. While both primary and metastatic NETs express SSTRs, primary breast tumors may also exhibit an uptake of 68Ga-somatostatin analogs, making the differential diagnosis between primary breast tumors and neuroendocrine metastases challenging. Additionally, imaging characteristics of breast metastases from NETs are poorly documented in the literature, posing a diagnostic challenge that extends to pathology, particularly when in the absence of clinical suspicion. Misdiagnosis in such cases can lead to inappropriate therapeutic interventions. We report the case of a 75-year-old female patient with a history of pancreatic NET who presented to our breast clinic for further evaluation of a breast mass after a PET-CT scan revealed moderate 68Ga-DOTATOC uptake. Multimodality breast examination, including mammography and multiparametric US with B-mode, Color Doppler, Strain Elastography (SE), Shear Wave Elastography (SWE), and contrast-enhanced US (CEUS), was performed. Following a core biopsy, the lesion underwent surgical excision, revealing the diagnosis of NET metastasis. This case highlights a rare instance of neuroendocrine tumor metastasis to the breast, assessed using various ultrasound techniques, with detailed imaging and quantitative analysis. The comprehensive multimodal assessment contributes to the limited body of literature and provides elements for the differential diagnosis of a rare breast lesion that should always be considered in the presence of a known primary NET.
Keywords: 68Ga-DOTATOC PET-CT; breast metastases; contrast-enhanced ultrasound (CEUS); differential diagnosis; misdiagnosis; multimodality ultrasound; neuroendocrine tumors (NETs); pancreatic NET; shear wave elastography (SWE); somatostatin receptor imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources

