Modulation of the Toll-like Receptor Pathway in Ovine Endometria During Early Pregnancy
- PMID: 40218311
- PMCID: PMC11987747
- DOI: 10.3390/ani15070917
Modulation of the Toll-like Receptor Pathway in Ovine Endometria During Early Pregnancy
Abstract
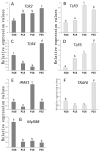
Correct immunological dialogue between the maternal uterus and conceptus is essential during implantation, and Toll-like receptors (TLRs) participate in maternal immune tolerance during pregnancy. This study aimed to analyze the effect of early pregnancy on Toll-like receptor pathways in the ovine endometrium. Ovine endometria were obtained on day 16 of the estrous cycle (N16) and days 13, 16, and 25 of pregnancy (P13, P16, and P25), and expression profiles of TLR members, including TLRs, tumor necrosis factor receptor-associated factor 6 (TRAF6), interleukin 1 receptor-associated kinase 1 (IRAK1), and myeloid differentiation primary response gene 88 (MyD88), were detected by quantitative real-time PCR, Western blot analysis, and immunohistochemistry analysis. The data of this study showed that the expression of TLR2 and TLR5 was gradually increased during early pregnancy compared to N16, and TLR3 expression was greater at P16 and P25 than at N16 and P13. However, the expression levels of TLR4 and TRAF6 were weaker at P13 and P16, and the expression of MyD88 was inhibited by early pregnancy. Furthermore, early pregnancy regulated IRAK1 expression. These findings corroborated that the TLR pathway was modulated in the ovine endometrium during early pregnancy, which may be involved in maternal immunoregulation.
Keywords: endometrium; pregnancy; sheep; toll-like receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Pregnancy-induced changes in the toll-like receptor pathway in the ovine duodenum.Vet Immunol Immunopathol. 2025 Apr;282:110900. doi: 10.1016/j.vetimm.2025.110900. Epub 2025 Feb 17. Vet Immunol Immunopathol. 2025. PMID: 39985901
-
Early pregnancy affects expression of Toll-like receptor signaling members in ovine spleen.Anim Reprod. 2021 Aug 6;18(2):e20210009. doi: 10.1590/1984-3143-AR2021-0009. eCollection 2021. Anim Reprod. 2021. PMID: 34394755 Free PMC article.
-
Early pregnancy affects the expression of toll-like receptor pathway in ovine thymus.Reprod Biol. 2020 Dec;20(4):547-554. doi: 10.1016/j.repbio.2020.10.003. Epub 2020 Nov 4. Reprod Biol. 2020. PMID: 33158780
-
Toll-like receptor family and signalling pathway.Biochem Soc Trans. 2000 Oct;28(5):563-6. doi: 10.1042/bst0280563. Biochem Soc Trans. 2000. PMID: 11044375 Review.
-
MicroRNA in TLR signaling and endotoxin tolerance.Cell Mol Immunol. 2011 Sep;8(5):388-403. doi: 10.1038/cmi.2011.26. Epub 2011 Aug 8. Cell Mol Immunol. 2011. PMID: 21822296 Free PMC article. Review.
References
-
- Marozio L., Nuzzo A.M., Gullo E., Moretti L., Canuto E.M., Tancredi A., Goia M., Cosma S., Revelli A., Rolfo A., et al. Immune checkpoints in recurrent pregnancy loss: New insights into a detrimental and elusive disorder. Int. J. Mol. Sci. 2023;24:13071. doi: 10.3390/ijms241713071. - DOI - PMC - PubMed
-
- Campanile G., Baruselli P.S., Limone A., D’Occhio M.J. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation—Implications for embryo survival in cattle: A review. Theriogenology. 2021;167:1–12. doi: 10.1016/j.theriogenology.2021.02.020. - DOI - PubMed
-
- Yang Q., Liu J., Wang Y., Zhao W., Wang W., Cui J., Yang J., Yue Y., Zhang S., Chu M., et al. A proteomic atlas of ligand-receptor interactions at the ovine maternal-fetal interface reveals the role of histone lactylation in uterine remodeling. J. Biol. Chem. 2022;298:101456. doi: 10.1016/j.jbc.2021.101456. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

