Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source
- PMID: 40218440
- PMCID: PMC11988020
- DOI: 10.3390/ani15071047
Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source
Abstract
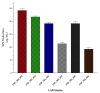
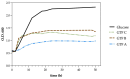
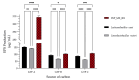
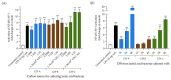
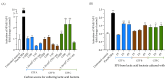
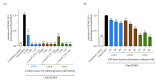
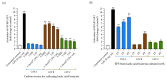
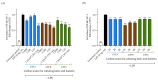
Exopolysaccharides (EPSs) produced by probiotic bacteria have garnered attention due to their effects on the gut health of humans and animals. The nutrients that probiotics access during their growth are essential for producing beneficial effects on host health. Direct immunomodulatory effects of graminan-type fructans (GTFs) from Agave tequilana through toll-like receptors (TLRs) have been demonstrated. However, the immunomodulatory effects of these fructans, mediated through the EPSs produced by the probiotics cultivated with them, remain unexplored. We explored the immunomodulatory effects of lactic acid bacteria (LAB) strains isolated from swine and their EPSs, based on the GTFs used as carbon sources during their growth. While the LAB strains activated the NF-κB pathway independently of the GTF source, their EPSs activated it in a GTF source-dependent manner. LAB activation through TLR2 showed a GTF source dependency, whereas their EPSs activated TLR2 independently of the GTF source. The LAB and their EPSs activated TLR4 in a GTF source-dependent manner. Both the LAB and their EPSs inhibited the activation of TLR2 and TLR4 agonists, which exhibited a strong dependence on the GTF source. The strength of GTF C's immunomodulatory effects on LAB illustrates its specificity, its impact on the EPS structure, and its biological effects. Our results support the promising health benefits of this synbiotic model for swine health and lowering inflammation.
Keywords: TLRs; agave; exopolysaccharides; fructans; lactobacilli; probiotics; swine.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
β(2→6)-Type fructans attenuate proinflammatory responses in a structure dependent fashion via Toll-like receptors.Carbohydr Polym. 2022 Feb 1;277:118893. doi: 10.1016/j.carbpol.2021.118893. Epub 2021 Nov 15. Carbohydr Polym. 2022. PMID: 34893295
-
Biofilm formation by agave epiphytic lactic acid bacteria fed with agave fructans.World J Microbiol Biotechnol. 2023 Sep 5;39(11):299. doi: 10.1007/s11274-023-03749-3. World J Microbiol Biotechnol. 2023. PMID: 37667093
-
Biological activities and applications of exopolysaccharides produced by lactic acid bacteria: a mini-review.World J Microbiol Biotechnol. 2023 Apr 11;39(6):155. doi: 10.1007/s11274-023-03610-7. World J Microbiol Biotechnol. 2023. PMID: 37039945 Review.
-
Prebiotic effect of fructans from Agave salmiana on probiotic lactic acid bacteria and in children as a supplement for malnutrition.Food Funct. 2022 Apr 4;13(7):4184-4193. doi: 10.1039/d1fo03852d. Food Funct. 2022. PMID: 35322820
-
Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells.Microorganisms. 2016 Aug 15;4(3):27. doi: 10.3390/microorganisms4030027. Microorganisms. 2016. PMID: 27681921 Free PMC article. Review.
Cited by
-
The Role of Microbial Exopolysaccharides in Preventing and Treating Cardiovascular Diseases.Microorganisms. 2025 Jun 29;13(7):1522. doi: 10.3390/microorganisms13071522. Microorganisms. 2025. PMID: 40732030 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

