Dynamic Interference Testing-Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices
- PMID: 40218498
- PMCID: PMC11991141
- DOI: 10.3390/s25071985
Dynamic Interference Testing-Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices
Abstract
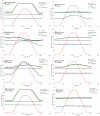
Background: Sensors for continuous glucose monitoring (CGM) are now commonly used by people with type 1 and type 2 diabetes. However, the response of these devices to potentially interfering nutritional, pharmaceutical, or endogenous substances is barely explored. We previously developed an in vitro test method for continuous and dynamic CGM interference testing and herein explore the sensitivity of the Abbott Libre2 (L2) and Dexcom G6 (G6) sensors to a panel of 68 individual substances.
Methods: In each interference experiment, L2 and G6 sensors were exposed in triplicate to substance gradients from zero to supraphysiological concentrations at a stable glucose concentration of 200 mg/dL. YSI Stat 2300 Plus was used as the glucose reference method. Interference was presumed if the CGM sensors showed a mean bias of at least ±10% from baseline with a tested substance at any given substance concentration.
Results: Both L2 and G6 sensors showed interference with the following substances: dithiothreitol (maximal bias from baseline: L2/G6: +46%/-18%), galactose (>+100%/+17%), mannose (>+100%/+20%), and N-acetyl-cysteine (+11%/+18%). The following substances were found to interfere with L2 sensors only: ascorbic acid (+48%), ibuprofen (+14%), icodextrin (+10%), methyldopa (+16%), red wine (+12%), and xylose (>+100%). On the other hand, the following substances were found to interfere with G6 sensors only: acetaminophen (>+100%), ethyl alcohol (+12%), gentisic acid (+18%), hydroxyurea (>+100%), l-cysteine (-25%), l-Dopa (+11%), and uric acid (+33%). Additionally, G6 sensors could subsequently not be calibrated for use after exposure to dithiothreitol, gentisic acid, l-cysteine, and mesalazine (sensor fouling).
Conclusions: Our standardized dynamic interference testing protocol identified several nutritional, pharmaceutical and endogenous substances that substantially influenced L2 and G6 sensor signals. Clinical trials are now necessary to investigate whether our findings are of relevance during routine care.
Keywords: Abbott Libre 2; Dexcom G6; continuous glucose monitoring; dynamic interference testing; interferents.
Conflict of interest statement
Authors Steven Setford and Mike Grady were employed by the LifeScan Scotland Ltd. company. Author Elizabeth Holt was employed by the LifeScan Global Corp. company. Author Andreas Pfützner has received consulting fees and travel support from Lifescan Global Corp. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Camerlingo N., Vettoretti M., Sparacino G., Facchinetti A., Mader J.K., Choudhary P., Del Favero S., Hypo-RESOLVE Consortium Choosing the duration of continuous glucose monitoring for reliable assessment of time in range: A new analytical approach to overcome the limitations of correlation-based methods. Diabet Med. 2022;39:e14758. - PubMed
-
- Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63:242–252. - PubMed
-
- Maiorino M.I., Signoriello S., Maio A., Chiodini P., Bellastella G., Scappaticcio L., Longo M., Giugliano D., Esposito K. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2020;43:1146–1156. - PubMed
-
- Beck R.W., Riddlesworth T., Ruedy K., Ahmann A., Bergenstal R., Haller S., Kollman C., Kruger D., McGill J.B., Polonsky W., et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA. 2017;317:371–378. doi: 10.1001/jama.2016.19975. - DOI - PubMed
-
- Heinemann L., Guido Freckmann G., Gabriele Faber-Heinemann G., Faber-Heinemann G., Guerra S., Waldenmaier D., Hermanns N. Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: Results of the multicentre, randomised controlled HypoDE study. Lancet. 2018;391:1367–1377. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

