Fabrication and Characterization of a Flexible Non-Enzymatic Electrochemical Glucose Sensor Using a Cu Nanoparticle/Laser-Induced Graphene Fiber/Porous Laser-Induced Graphene Network Electrode
- PMID: 40218852
- PMCID: PMC11991655
- DOI: 10.3390/s25072341
Fabrication and Characterization of a Flexible Non-Enzymatic Electrochemical Glucose Sensor Using a Cu Nanoparticle/Laser-Induced Graphene Fiber/Porous Laser-Induced Graphene Network Electrode
Abstract
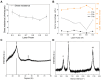
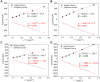
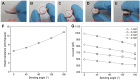
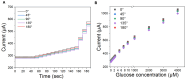
We demonstrate a flexible electrochemical biosensor for non-enzymatic glucose detection under different bending conditions. The novel flexible glucose sensor consists of a Cu nanoparticle (NP)/laser-induced graphene fiber (LIGF)/porous laser-induced graphene (LIG) network structure on a polyimide film. The bare LIGF/LIG electrode fabricated using an 8.9 W laser power shows a measured sheet resistance and thickness of 6.8 Ω/□ and ~420 μm, respectively. In addition, a conventional Cu NP electroplating method is used to fabricate a Cu/LIGF/LIG electrode-based glucose sensor that shows excellent glucose detection characteristics, including a sensitivity of 1438.8 µA/mM∙cm2, a limit of detection (LOD) of 124 nM, and a broad linear range at an applied potential of +600 mV. Significantly, the Cu/LIGF/LIG electrode-based glucose sensor exhibits a relatively high sensitivity, low LOD, good linear detection range, and long-term stability at bending angles of 0°, 45°, 90°, 135°, and 180°.
Keywords: Cu nanoparticle; flexible sensor; laser-induced graphene; non-enzymatic electrochemical detection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration.Biosens Bioelectron. 2020 Jul 15;160:112220. doi: 10.1016/j.bios.2020.112220. Epub 2020 Apr 20. Biosens Bioelectron. 2020. PMID: 32339151
-
Laser-induced graphene electrode modified by platinum nanoparticle/zein/gelatin/glucose oxidase for non-invasive glucose sensor in multiple biofluids.Anal Chim Acta. 2025 Jun 1;1353:343974. doi: 10.1016/j.aca.2025.343974. Epub 2025 Mar 25. Anal Chim Acta. 2025. PMID: 40221209
-
Improved mechanical stability of laser-induced graphene electrodes in enzyme-free glucose sensors through transferring to PDMS and partial PDMS etching.Mikrochim Acta. 2025 Aug 2;192(8):550. doi: 10.1007/s00604-025-07390-9. Mikrochim Acta. 2025. PMID: 40753275
-
A performance improvement of enzyme-based electrochemical lactate sensor fabricated by electroplating novel PdCu mediator on a laser induced graphene electrode.Bioelectrochemistry. 2022 Dec;148:108259. doi: 10.1016/j.bioelechem.2022.108259. Epub 2022 Sep 10. Bioelectrochemistry. 2022. PMID: 36179392
-
Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor.Molecules. 2024 Nov 29;29(23):5662. doi: 10.3390/molecules29235662. Molecules. 2024. PMID: 39683820 Free PMC article.
Cited by
-
The Role of Nanomaterials in the Wearable Electrochemical Glucose Biosensors for Diabetes Management.Biosensors (Basel). 2025 Jul 14;15(7):451. doi: 10.3390/bios15070451. Biosensors (Basel). 2025. PMID: 40710101 Free PMC article. Review.
References
-
- He W., Sun Y., Xi J., Abdurhman A.A.M., Ren J., Duan H. Printing graphene-carbon nanotube-ionic liquid gel on graphene paper: Towards flexible electrodes with efficient loading of PtAu alloy nanoparticles for electrochemical sensing of blood glucose. Anal. Chim. Acta. 2016;903:61–68. doi: 10.1016/j.aca.2015.11.019. - DOI - PubMed
-
- Munje R.D., Muthukumar S., Prasad S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017;238:482–490. doi: 10.1016/j.snb.2016.07.088. - DOI
-
- Huang C., Hao Z., Qi T., Pan Y., Zhao X. An integrated flexible and reusable graphene field effect transistor nanosensor for monitoring glucose. J. Mater. 2020;6:308–314. doi: 10.1016/j.jmat.2020.02.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

