Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer's Mice: A Pilot Experimental Study
- PMID: 40218866
- PMCID: PMC11990804
- DOI: 10.3390/nu17071108
Omega-3 EPA Supplementation Shapes the Gut Microbiota Composition and Reduces Major Histocompatibility Complex Class II in Aged Wild-Type and APP/PS1 Alzheimer's Mice: A Pilot Experimental Study
Abstract
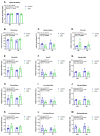
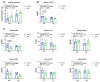
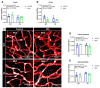
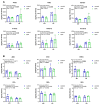
Background/Objectives: Neuroinflammation, a hallmark of Alzheimer's disease (AD), is characterized by elevated levels of inflammatory signaling molecules, including cytokines and eicosanoids, as well as increased microglial reactivity, and is augmented by gut microbiota dysbiosis via the gut-brain axis. We conducted a pilot experiment to elucidate the anti-inflammatory effects of dietary omega-3 polyunsaturated fatty acid (ω-3 PUFA) eicosapentaenoic acid (EPA) on the gut microbiota and neuroinflammation. Methods: Female APP/PS1 mice (TG) and non-transgenic littermates (WT), 13-14 months old, were fed a diet supplemented with 0.3% EPA or control chow for 3 weeks. The gut microbiota composition, hippocampal and plasma eicosanoids levels, platelet activation, and microglial phagocytosis, as well as the brain and retinal genes and protein expression, were analyzed. Results: EPA supplementation decreased the percentage of Bacteroidetes and increased bacteria of the phylum Firmicutes in APP/PS1 and WT mice. Inflammatory lipid mediators were elevated in the hippocampus of the TG mice, accompanied by a reduction in the endocannabinoid docosahexaenoyl ethanolamide (DHEA). Dietary EPA did not affect hippocampal lipid mediators, but reduced the levels of arachidonic-derived 5-HETE and N-arachidonoylethanolamine (AEA) in WT plasma. Moreover, EPA supplementation decreased major histocompatibility complex class II (MHCII) gene expression in the retina in both genotypes, and MHCII+ cells in the hippocampus of TG mice. Conclusions: This pilot study showed that short-term EPA supplementation shaped the gut microbiota by increasing butyrate-producing bacteria of the Firmicutes phylum and decreasing Gram-negative LPS-producing bacteria of the Bacteroidetes phylum, and downregulated the inflammatory microglial marker MHCII in two distinct regions of the central nervous system (CNS). Further investigation is needed to determine whether EPA-mediated effects on the microbiome and microglial MHCII have beneficial long-term effects on AD pathology and cognition.
Keywords: APP/PS1; Alzheimer’s disease; MHCII; eicosanoids; eicosapentaenoic acid (EPA); gut microbiota; lipid droplets; microglia; omega-3 polyunsaturated fatty acids; phagocytosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
High-fat diet alters stress behavior, inflammatory parameters and gut microbiota in Tg APP mice in a sex-specific manner.Neurobiol Dis. 2021 Nov;159:105495. doi: 10.1016/j.nbd.2021.105495. Epub 2021 Aug 31. Neurobiol Dis. 2021. PMID: 34478848
-
Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration.Eur J Nutr. 2018 Aug;57(5):1781-1791. doi: 10.1007/s00394-017-1462-7. Epub 2017 May 18. Eur J Nutr. 2018. PMID: 28523372
-
A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice.Neuroscience. 2007 Oct 26;149(2):286-302. doi: 10.1016/j.neuroscience.2007.08.018. Epub 2007 Aug 14. Neuroscience. 2007. PMID: 17904756
-
Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease.Life Sci. 2021 Jan 1;264:118627. doi: 10.1016/j.lfs.2020.118627. Epub 2020 Oct 22. Life Sci. 2021. PMID: 33169684 Review.
-
Impact of Omega-3 Fatty Acids on the Gut Microbiota.Int J Mol Sci. 2017 Dec 7;18(12):2645. doi: 10.3390/ijms18122645. Int J Mol Sci. 2017. PMID: 29215589 Free PMC article. Review.
Cited by
-
MIND Diet and Hippocampal Sclerosis Among Community-Based Older Adults.JAMA Netw Open. 2025 Aug 1;8(8):e2526089. doi: 10.1001/jamanetworkopen.2025.26089. JAMA Netw Open. 2025. PMID: 40773193 Free PMC article.
References
-
- World Alzheimer Report 2024: Global Changes in Attitudes to Dementia. Alzheimer’s Disease International; London, UK: 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

