Can Iron Absorption in Molasses Be Increased with Probiotic Additives? "Molasses with Increased Bioavailability"
- PMID: 40218909
- PMCID: PMC11990919
- DOI: 10.3390/nu17071150
Can Iron Absorption in Molasses Be Increased with Probiotic Additives? "Molasses with Increased Bioavailability"
Abstract
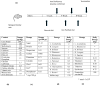
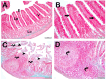
Introduction: There are many studies on the chemical and enzymatic interactions of probiotics, and the effects of Lactobacillus plantarum 299v on iron absorption have been clearly shown. The aim of this study was to investigate the effect of probiotics on the absorption of iron in molasses. Material and method: Wistar rats (n = 46) were taken four weeks after birth and divided into seven groups. Iron deficiency anemia was induced by giving "iron purified pellet" to the groups except the control group for four weeks and then the groups were given nutrients for eight weeks. In addition to iron deficiency anemia tests, immunohistochemical markers such as SCL11a, IRE1, Wnt2, and CD71 were examined. Results: The mean weight of the subjects was 309.5 ± 63.9 (226-424) g and no significant difference was observed in the laboratory values of metabolic data. When the laboratory values of iron deficiency anemia were examined, a statistically significant difference was found between the mean ferritin (p = 0.03) and hepcidin (p = 0.02) values of the groups. Discussion: Iron absorption analysis values were generally higher in the group receiving Fe3+ as expected. However, when the groups receiving molasses and additives were compared, the highest plasma iron level and Hb value were found in the Lactobacillus plantarum 299v group, and the highest ferritin and hepcidin levels were found in the Multiprobiotic group. No difference was observed between the body weights and fasting serum glucose levels of the groups despite daily molasses consumption, indicating the metabolic proactive effects of probiotics. Conclusions: Although no significant difference was detected between the groups receiving probiotics, iron absorption in molasses was increased with probiotic supplementation.
Keywords: iron absorption; molasses; prebiotic; probiotic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: a double-isotope cross-over single-blind study in women of reproductive age.Br J Nutr. 2015 Oct 28;114(8):1195-202. doi: 10.1017/S000711451500241X. Br J Nutr. 2015. PMID: 26428277 Free PMC article. Clinical Trial.
-
The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial.Nutrients. 2020 Apr 30;12(5):1279. doi: 10.3390/nu12051279. Nutrients. 2020. PMID: 32365981 Free PMC article. Clinical Trial.
-
Efficacy of probiotic, prebiotic, and synbiotics supplements in individuals with anemia: a systematic review and meta-analysis of randomized controlled trials.BMC Gastroenterol. 2024 Dec 23;24(1):472. doi: 10.1186/s12876-024-03562-8. BMC Gastroenterol. 2024. PMID: 39716076 Free PMC article.
-
Hepcidin and Erythroferrone Correlate with Hepatic Iron Transporters in Rats Supplemented with Multispecies Probiotics.Molecules. 2020 Apr 5;25(7):1674. doi: 10.3390/molecules25071674. Molecules. 2020. PMID: 32260496 Free PMC article.
-
A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status.Nutrients. 2019 Dec 3;11(12):2938. doi: 10.3390/nu11122938. Nutrients. 2019. PMID: 31816981 Free PMC article.
References
-
- Özbey A., Öncül N., Erdoğan K., Yıldırım Z., Yıldırım M. Some Physical, Chemical and Microbiological Properties of Çalma Pekmez Produced in Tokat Region, Turkey. [(accessed on 23 December 2023)];Akad. Gıda. 2013 11:46–52. Available online: https://dergipark.org.tr/tr/pub/akademik-gida/issue/55794/763795.
-
- Akbulut M., Özcan M. Some Physical, Chemical, and Rheological Properties of Sweet Sorghum (Sorghum bicolor (L) Moench) Pekmez (Molasses) Int. J. Food Prop. 2008;11:79–91. doi: 10.1080/10942910701233389. - DOI
-
- Salık M.A., Arslaner A., Çakmakçı S. Determination of Some Physical, Chemical and Antioxidant Properties of Erzincan Traditional Mulberry Pekmez (Molasses) Turk. J. Agric. Food Sci. Technol. 2021;9:181–190. doi: 10.24925/turjaf.v9i1.181-190.3802. - DOI
-
- Cifci A., Özkan M. Iron physiopathology and approach to iron deficiency anemia: New treatment strategies. J. Health Sci. Med. 2018;1:40–44. doi: 10.32322/jhsm.430073. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

