Dual Action of Pueraria montana var. lobata Extract on Myogenesis and Muscle Atrophy
- PMID: 40218975
- PMCID: PMC11990788
- DOI: 10.3390/nu17071217
Dual Action of Pueraria montana var. lobata Extract on Myogenesis and Muscle Atrophy
Abstract
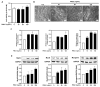
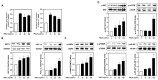
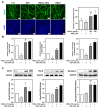
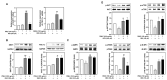
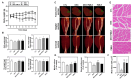
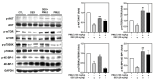
Background/Objectives: Muscle atrophy, defined by diminished muscle mass and function, is a notable concern associated with aging, disease, and glucocorticoid treatment. Pueraria montana var. lobata extract (PMLE) demonstrates multiple bioactive properties, such as antioxidant, anti-inflammatory, and metabolic regulatory activities; however, its role in muscle atrophy has not been extensively investigated to date. This study examined how PMLE influences both muscle cell differentiation and dexamethasone (DEX)-induced muscle degeneration by focusing on the underlying molecular mechanisms. Methods: This study examined the effects of PMLE on myogenic differentiation and DEX-induced muscle atrophy. C2C12 myoblasts were treated with PMLE (10-100 ng/mL) and assessed for changes in the expression of myogenesis-related genes and activation of Akt/mTOR and AMPK/SIRT1/PGC-1α signaling cascades. In vivo, a DEX-induced muscle atrophy model was used to assess muscle mass, fiber morphology, and molecular changes. Results: PMLE PMLE promoted muscle cell development by increasing the expression of MyHC, MyoD, and myogenin while activating protein synthesis and mitochondrial biogenesis pathways. PMLE counteracted DEX-induced myotube atrophy, restoring myotube diameter and promoting cellular fusion in vitro. In vivo, PMLE mitigated muscle degradation in fast-twitch muscle groups and reversed DEX-induced suppression of key anabolic and mitochondrial pathways. Conclusions: These findings suggest that PMLE promotes myogenic differentiation and protects against muscle atrophy by regulating critical molecular pathways, indicating its promise as a treatment candidate for conditions involving muscle wasting. Further studies are required to assess its clinical application and long-term safety efficacy.
Keywords: Pueraria montana var. lobata extract; dexamethasone; mitochondrial biogenesis; muscle atrophy; protein synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo.Nutrients. 2024 Aug 13;16(16):2687. doi: 10.3390/nu16162687. Nutrients. 2024. PMID: 39203823 Free PMC article.
-
The Extract of Gloiopeltis tenax Enhances Myogenesis and Alleviates Dexamethasone-Induced Muscle Atrophy.Int J Mol Sci. 2024 Jun 20;25(12):6806. doi: 10.3390/ijms25126806. Int J Mol Sci. 2024. PMID: 38928510 Free PMC article.
-
Saururus chinensis (Lour.) Baill. extract promotes skeletal muscle cell differentiation by positively regulating mitochondrial biogenesis and AKT/mTOR signaling in vitro.Mol Med Rep. 2024 Jul;30(1):125. doi: 10.3892/mmr.2024.13250. Epub 2024 May 24. Mol Med Rep. 2024. PMID: 38785149 Free PMC article.
-
Corticosteroids and muscle wasting: role of transcription factors, nuclear cofactors, and hyperacetylation.Curr Opin Clin Nutr Metab Care. 2010 Jul;13(4):423-8. doi: 10.1097/MCO.0b013e32833a5107. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20473154 Free PMC article. Review.
-
Cisplatin-Induced Muscle Wasting and Atrophy: Molecular Mechanism and Potential Therapeutic Interventions.J Cachexia Sarcopenia Muscle. 2025 Jun;16(3):e13817. doi: 10.1002/jcsm.13817. J Cachexia Sarcopenia Muscle. 2025. PMID: 40343378 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

