Abilities of Rare Sugar Members to Release Glucagon-like Peptide-1 and Suppress Food Intake in Mice
- PMID: 40218979
- PMCID: PMC11990814
- DOI: 10.3390/nu17071221
Abilities of Rare Sugar Members to Release Glucagon-like Peptide-1 and Suppress Food Intake in Mice
Abstract
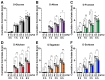
Background/Objectives: Rare sugars, which naturally exist in small quantities, have gained attention as next-generation functional sugars due to their sweetness and low calorie content. Some of them have already been commercialized. Rare sugar-containing syrups, produced through alkaline isomerization of high-fructose corn syrup, are effective in preventing obesity and type 2 diabetes. However, the mechanisms underlying these effects remain incompletely understood. Recently, D-allulose has been found to improve hyperphagic obesity by stimulating the secretion of the intestinal hormone glucagon-like peptide-1 (GLP-1). The present study aimed to determine the comparative effects of aldohexoses (D-glucose, D-allose) and ketohexoses (D-fructose, D-allulose, D-tagatose, D-sorbose) on GLP-1 secretion and food intake in male mice. Method and Results: Single peroral administration of four ketohexoses at 1 and 3 g/kg, but not aldohexoses at 1 and 3 g/kg, significantly increased plasma GLP-1 concentrations with comparable efficacy. Moreover, these ketohexoses at 1 g/kg suppressed food intake in the short term, an effect blunted by GLP-1 receptor antagonism. In contrast, zero-calorie D-allose at 3 g/kg suppressed feeding without raising plasma GLP-1 levels. Conclusions: These results demonstrate that D-allulose, D-tagatose, and D-sorbose, which are low-calorie rare sugars classified as ketohexoses, suppress food intake through promoting GLP-1 secretion, showing their potential to prevent and/or ameliorate type 2 diabetes, obesity and related diseases.
Keywords: GLP-1; allose; allulose; food intake; fructose; rare sugars; sorbose; tagatose.
Conflict of interest statement
T.K. and T.Y. (Takako Yamada) were employed by Matsutani Chemical Industry Co., Ltd. Y.I. and T.Y. (Toshihiko Yada) declares that this study received funding from Matsutani Chemical Industry Co., Ltd. The funder provided rare sugars and was partly involved in designing and performing the experiments. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

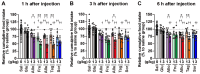

References
-
- Hayashi N., Yamada T., Takamine S., Iida T., Okuma K., Tokuda M. Weight Reducing Effect and Safety Evaluation of Rare Sugar Syrup by a Randomized Double-Blind, Parallel-Group Study in Human. J. Funct. Foods. 2014;11:152–159. doi: 10.1016/j.jff.2014.09.020. - DOI
-
- Yoshihara A., Kozakai T., Shintani T., Matsutani R., Ohtani K., Iida T., Akimitsu K., Izumori K., Gullapalli P.K. Purification and Characterization of D-Allulose 3-Epimerase Derived from Arthrobacter Globiformis M30, a GRAS Microorganism. J. Biosci. Bioeng. 2017;123:170–176. doi: 10.1016/j.jbiosc.2016.09.004. - DOI - PubMed
-
- Morimoto K., Park C.-S., Ozaki M., Takeshita K., Shimonishi T., Granström T.B., Takata G., Tokuda M., Izumori K. Large Scale Production of D-Allose from d-Psicose Using Continuous Bioreactor and Separation System. Enzyme Microb. Technol. 2006;38:855–859. doi: 10.1016/j.enzmictec.2005.08.014. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

