The Extended Synaptotagmins of Physcomitrium patens
- PMID: 40219095
- PMCID: PMC11990657
- DOI: 10.3390/plants14071027
The Extended Synaptotagmins of Physcomitrium patens
Abstract
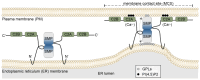
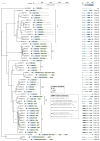
Membrane contact sites (MCSs) between the endoplasmic reticulum and the plasma membrane enable the transport of lipids without membrane fusion. Extended Synaptotagmins (ESYTs) act at MCSs, functioning as tethers between two membrane compartments. In plants, ESYTs have been mainly investigated in A. thaliana and shown to maintain the integrity of the plasma membrane, especially during stress responses like cold acclimatization, mechanical trauma, and salt stress. ESYTs are present at the MCSs of plasmodesmata, where they regulate defense responses by modulating cell-to-cell transfer of pathogens. Here, the analysis of ESYTs was expanded to the bryophyte Physcomitrium patens, an extant representative of the earliest land plant lineages. P. patens was found to contain a large number of ESYTs, distributed over all previously established classes and an additional class not present in A. thaliana. Motif discovery identified regions in the Synaptotagmin-like mitochondrial (SMP) domain that may explain phylogenetic relationships as well as protein function. The adaptation mechanisms of P. patens necessary to conquer land and its simple tissue structure make it highly suitable as a model organism to study ESYT functions in tip growth, stress responses, and plasmodesmata-mediated transport, and open new directions of research regarding the function of MCSs in cellular processes and plant evolution.
Keywords: Physcomitrium patens; bryophytes; extended synaptotagmins; lipid transport proteins; membrane contact sites; plasmodesmata; tip growth.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Giordano F., Saheki Y., Idevall-Hagren O., Colombo S.F., Pirruccello M., Milosevic I., Gracheva E.O., Bagriantsev S.N., Borgese N., De Camilli P. PI(4,5)P2-Dependent and Ca2+-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. - DOI - PMC - PubMed
-
- Schapire A.L., Voigt B., Jasik J., Rosado A., Lopez-Cobollo R., Menzel D., Salinas J., Mancuso S., Valpuesta V., Baluska F., et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell. 2008;20:3374–3388. doi: 10.1105/tpc.108.063859. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

