Hydrogel-Based Bioinks for Coaxial and Triaxial Bioprinting: A Review of Material Properties, Printing Techniques, and Applications
- PMID: 40219306
- PMCID: PMC11991663
- DOI: 10.3390/polym17070917
Hydrogel-Based Bioinks for Coaxial and Triaxial Bioprinting: A Review of Material Properties, Printing Techniques, and Applications
Abstract
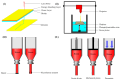
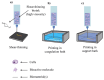
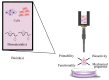
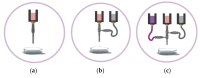
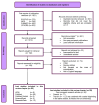
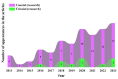
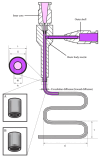
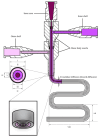
Three-dimensional bioprinting technology has emerged as a rapidly advancing multidisciplinary field with significant potential for tissue engineering applications. This technology enables the formation of complex tissues and organs by utilizing hydrogels, with or without cells, as scaffolds or structural supports. Among various bioprinting methods, advanced bioprinting using coaxial and triaxial nozzles stands out as a promising technique. Coaxial bioprinting technique simultaneously deposits two material streams through a coaxial nozzle, enabling controlled formation of an outer shell and inner core construct. In contrast, triaxial bioprinting utilizes three material streams namely the outer shell, inner shell and inner core to fabricate more complex constructs. Despite the growing interest in 3D bioprinting, the development of suitable cell-laden bioinks for creating complex tissues remains unclear. To address this gap, a systematic review was conducted using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart, collecting 1621 papers from various databases, including Web of Science, PUBMED, SCOPUS, and Springer Link. After careful selection, 85 research articles focusing on coaxial and triaxial bioprinting were included in the review. Specifically, 77 research articles concentrated on coaxial bioprinting and 11 focused on triaxial bioprinting, with 3 covering both techniques. The search, conducted between 1 April and 30 September 2023, had no restrictions on publication date, and no meta-analyses were carried out due to the heterogeneity of studies. The primary objective of this review is to assess and identify the most commonly occurring cell-laden bioinks critical for successful advancements in bioprinting technologies. Specifically, the review focuses on delineating the commonly explored bioinks utilized in coaxial and triaxial bioprinting approaches. It focuses on evaluating the inherent merits of these bioinks, systematically comparing them while emphasizing their classifications, essential attributes, properties, and potential limitations within the domain of tissue engineering. Additionally, the review considers the applications of these bioinks, offering comprehensive insights into their efficacy and utility in the field of bioprinting technology. Overall, this review provides a comprehensive overview of some conditions of the relevant hydrogel bioinks used for coaxial and triaxial bioprinting of tissue constructs. Future research directions aimed at advancing the field are also briefly discussed.
Keywords: bioprinting; coaxial and triaxial bioprinting; hydrogel bioinks; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Coaxial Bioprinting of Enzymatically Crosslinkable Hyaluronic Acid-Tyramine Bioinks for Tissue Regeneration.Polymers (Basel). 2024 Aug 30;16(17):2470. doi: 10.3390/polym16172470. Polymers (Basel). 2024. PMID: 39274103 Free PMC article.
-
3D Coaxial Bioprinting: Process Mechanisms, Bioinks and Applications.Prog Biomed Eng (Bristol). 2022 Apr;4(2):022003. doi: 10.1088/2516-1091/ac631c. Epub 2022 Apr 20. Prog Biomed Eng (Bristol). 2022. PMID: 35573639 Free PMC article.
-
Nanocomposite bioinks for 3D bioprinting.Acta Biomater. 2022 Oct 1;151:45-69. doi: 10.1016/j.actbio.2022.08.014. Epub 2022 Aug 13. Acta Biomater. 2022. PMID: 35970479 Review.
-
Candidate Bioinks for Extrusion 3D Bioprinting-A Systematic Review of the Literature.Front Bioeng Biotechnol. 2021 Oct 13;9:616753. doi: 10.3389/fbioe.2021.616753. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34722473 Free PMC article.
References
-
- Sanchez E., Gomez-Blanco J., Nieto E., Casado J., Macias-Garcia A., Diez M., Carrasco-Amador J., Martin D., Sanchez-Margallo F., Pagador J. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020;8:776. doi: 10.3389/fbioe.2020.00776. - DOI - PMC - PubMed
-
- Zhang Y. Ph.D. Thesis. Industrial Engineering in the Graduate College, University of Iowa; Iowa City, IA, USA: 2014. 3D Bioprinting of Vasculature Network for Tissue Engineering.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

