How Molar Mass, Acid Type, and Coagulation Bath Composition Influence Coagulation Kinetics, Mechanical Properties, and Swelling Behavior of Chitosan Filaments: A Full Factorial Approach
- PMID: 40219316
- PMCID: PMC11991260
- DOI: 10.3390/polym17070927
How Molar Mass, Acid Type, and Coagulation Bath Composition Influence Coagulation Kinetics, Mechanical Properties, and Swelling Behavior of Chitosan Filaments: A Full Factorial Approach
Abstract
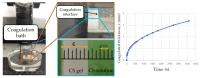
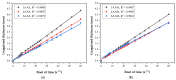
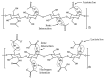
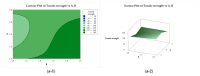
In this study, a full multilevel factorial design (21 × 31 × 21) × 2 was conducted to investigate the effects of molar mass of chitosan (CS), the type of acid used for dissolution, and the composition of the coagulation bath on the coagulation, mechanical properties, and swelling of the filaments. The results showed the statistical significance of the factors in the characteristics of these filaments. The coagulation followed Fick's second law of diffusion, with an increase in the chitosan molar mass reducing the coagulation rate, as did the use of acetic acid instead of lactic acid. CS with higher molar mass produced filaments with larger diameters, but without a proportional increase in tensile strength. Swelling was influenced by the acid and composition of the coagulation bath. The interaction of CS with acid and the CS molar mass factor were the terms of greatest statistical significance. Crystallinity was higher for samples dissolved in aqueous solutions of acetic acid and coagulated with ethanol, while lactic acid induced greater structural disorder. Samples coagulated with ethanol presented more homogeneous surfaces, while methanol resulted in rougher filaments. These findings emphasize the critical role of processing conditions in tailoring the properties of CS filaments, providing valuable insights for their optimization for biomedical applications.
Keywords: chitosan; coagulation process; full factorial design; mechanical properties; swelling behavior; wet spinning.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
















Similar articles
-
Wet-spun graphene filaments: effect of temperature of coagulation bath and type of reducing agents on mechanical & electrical properties.RSC Adv. 2018 May 14;8(31):17443-17452. doi: 10.1039/c8ra02325e. eCollection 2018 May 9. RSC Adv. 2018. PMID: 35539226 Free PMC article.
-
Chitosan Woven Meshes: Influence of Threads Configuration on Mechanical, Morphological, and Physiological Properties.Polymers (Basel). 2020 Dec 25;13(1):47. doi: 10.3390/polym13010047. Polymers (Basel). 2020. PMID: 33375542 Free PMC article.
-
New Solvent and Coagulating Agent for Development of Chitosan Fibers by Wet Spinning.Polymers (Basel). 2021 Jun 28;13(13):2121. doi: 10.3390/polym13132121. Polymers (Basel). 2021. PMID: 34203312 Free PMC article.
-
N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials.Materials (Basel). 2019 Jun 4;12(11):1807. doi: 10.3390/ma12111807. Materials (Basel). 2019. PMID: 31167371 Free PMC article.
-
Wet-Spun Chitosan-Sodium Caseinate Fibers for Biomedicine: From Spinning Process to Physical Properties.Int J Mol Sci. 2024 Feb 1;25(3):1768. doi: 10.3390/ijms25031768. Int J Mol Sci. 2024. PMID: 38339046 Free PMC article.
References
-
- Dutta P.K., Tripathi S., Mehrotra G.K., Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. - DOI
LinkOut - more resources
Full Text Sources

