[Observation of the efficacy of dupilumab for treatment of atopic dermatitis in the elderly]
- PMID: 40219560
- PMCID: PMC11992437
- DOI: 10.19723/j.issn.1671-167X.2025.02.012
[Observation of the efficacy of dupilumab for treatment of atopic dermatitis in the elderly]
Abstract
Objective: To evaluate the efficacy and safety of dupilumab in the treatment of atopic dermatitis in the elderly.
Methods: In this study, elderly patients with atopic dermatitis treated with dupilumab for at least 16 weeks in the Department of Dermatology and Venereology of Beijing Anzhen Hospital from January 2021 to October 2023 were retrospectively collected. Clinical indicators were compared before, during and after treatment, including pruritus numerical rating score (PNRS), eczema area and severity index (EASI), dermatology life quality index (DLQI) score, and the incidence of adverse events was recorded. The expression of interferon γ (IFN-γ), interleukin-4 (IL-4) and interleukin-6 (IL-6) in peripheral blood were compared before treatment and after 16 weeks of treatment.
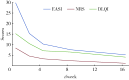
Results: A total of 90 elder patients with atopic dermatitis were included, EASI, PNRS and DLQI scores all showed a gradual downward trend during the treatment period, which was manifested as a rapid decline in the first 4 weeks after starting treatment, and then the decline gradually leveled off. The results of point-to-point comparison showed that EASI, PNRS and DLQI scores in 4 weeks after treatment were significantly lower than those before treatment (P < 0.001); In the 16th week after treatment, the scores of the above therapeutic indicators were further reduced, and the difference was statistically significant compared with the 4th week (P < 0.01); The EASI score was significantly lower at each time point than the previous time point, indicating that the patients' skin lesions continued to improve significantly. The overall efficacy of dupliumab was evaluated. After 4 weeks of treatment, 62.89% of patients achieved EASI-50 (EASI score decreased by ≥50%), and 74.4% of patients' DLQI score decreased by ≥4 points. After 16 weeks of treatment, 57.8% of the patients achieved EASI-75, 32.2% achieved EASI-90, and the PNRS and DLQI scores of all the patients decreased by ≥4 points. After 16 weeks of treatment, the expression levels of IL-4 and IL-6 were (31.62±6.23) ng/L and (14.36±2.25) ng/L, respectively, which were significantly lower than those before treatment (P < 0.001), and the expression level of IFN-γ was (15.37±3.14) ng/L, which was higher than before treatment (P < 0.001).The main adverse reactions were conjunctivitis (2 cases), injection site reaction (3 cases) and multiple bacterial folliculitis of the back (2 cases), which could be alleviated by symptomatic treatment, and no serious adverse reactions occurred.
Conclusion: Dupilumab has shown good efficacy in the treatment of elderly atopic dermatitis, which can effectively improve clinical symptoms such as itching and skin lesions, improve the quality of life of patients, and no serious adverse reactions occurred during treatment, so it is safe and worthy of clinical promotion.
目的: 观察度普利尤单抗治疗老年特应性皮炎(atopic dermatitis, AD)的疗效及安全性。
方法: 回顾性收集北京安贞医院皮肤性病科2021年1月至2023年10月收治的接受度普利尤单抗治疗至少16周的老年AD患者, 比较治疗前、治疗期间以及治疗后的临床指标, 包括瘙痒数字评价量表(pruritus numerical rating score, PNRS)、湿疹面积和严重程度指数(eczema area and severity index, EASI)、皮肤病生活质量指数(dermatology life quality index, DLQI), 同时记录发生的不良反应; 比较治疗前及治疗16周后外周血干扰素γ (interferon-γ, IFN-γ)、白细胞介素(interleukin, IL)-4、IL-6表达情况。
结果: 共90例老年性特应性皮炎患者纳入本研究, EASI、PNRS、DLQI评分在治疗期间均呈下降趋势, 具体表现为在启动治疗后的前4周内快速下降, 随后下降逐渐趋于平缓。两两时间点比较结果表明, 治疗后第4周EASI、PNRS和DLQI评分均较治疗前明显降低(P<0.001); 治疗后第16周时, 以上疗效指标评分均进一步降低, 与第4周相比差异有统计学意义(P<0.01), 其中EASI评分在各个时间点均比前一时间点明显降低, 表明患者皮损持续明显改善。对度普利尤单抗的总体疗效进行评价, 治疗4周时有62.89%的患者达到EASI-50(即EASI评分下降≥50%), 74.4%的患者DLQI评分下降≥4分; 16周时有57.8%的患者达到EASI-75, 32.2%的患者达到EASI-90, 且所有患者的PNRS、DLQI评分均下降≥4分。外周血炎症指标表达水平检测结果表明, 治疗16周后IL-4、IL-6表达水平分别为(31.62±6.23) ng/L、(14.36±2.25) ng/L, 均较治疗前明显降低(P<0.001), 而IFN-γ的表达水平为(15.37±3.14) ng/L, 较治疗前上升(P<0.001)。不良反应主要为结膜炎(2例)、注射部位反应(3例)及背部多发性细菌性毛囊炎(2例), 对症治疗均可缓解, 未出现严重不良反应。
结论: 度普利尤单抗在老年AD治疗中表现出良好的疗效, 可有效改善瘙痒、皮损等临床症状, 并能提高患者生活质量, 治疗期间未出现严重的不良反应, 安全性较好, 值得临床推广。
Keywords: Atopic dermatitis; Dupilumab; Inflammatory response; Old age.
Conflict of interest statement
Figures
Similar articles
-
A prospective observational cohort study comparing the treatment effectiveness and safety of ciclosporin, dupilumab and methotrexate in adult and paediatric patients with atopic dermatitis: results from the UK-Irish A-STAR register.Br J Dermatol. 2024 Nov 18;191(6):988-999. doi: 10.1093/bjd/ljae287. Br J Dermatol. 2024. PMID: 39044673
-
Efficacy and Safety of Abrocitinib in Patients with Severe and/or Difficult-to-Treat Atopic Dermatitis: A Post Hoc Analysis of the Randomized Phase 3 JADE COMPARE Trial.Am J Clin Dermatol. 2023 Jul;24(4):609-621. doi: 10.1007/s40257-023-00785-5. Epub 2023 May 22. Am J Clin Dermatol. 2023. PMID: 37213005 Free PMC article. Clinical Trial.
-
Efficacy and safety of upadacitinib versus dupilumab in adults and adolescents with moderate-to-severe atopic dermatitis: week 16 results of an open-label randomized efficacy assessor-blinded head-to-head phase IIIb/IV study (Level Up).Br J Dermatol. 2024 Dec 23;192(1):36-45. doi: 10.1093/bjd/ljae404. Br J Dermatol. 2024. PMID: 39438067 Clinical Trial.
-
Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis.J Am Acad Dermatol. 2021 Jan;84(1):139-147. doi: 10.1016/j.jaad.2020.08.051. Epub 2020 Aug 18. J Am Acad Dermatol. 2021. PMID: 32822798
-
Effectiveness and safety of systemic therapy for moderate-to-severe atopic dermatitis in children and adolescent patients: a systematic review.Front Immunol. 2024 May 15;15:1367099. doi: 10.3389/fimmu.2024.1367099. eCollection 2024. Front Immunol. 2024. PMID: 38812522 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous