Rosmarinus officinalis Ethanolic Extracts Rescues BV-2 Cells via Modulating Inflammation and Redox Balance: Comparative Study With Carnosol and Carnosic Acid
- PMID: 40219627
- PMCID: PMC11992469
- DOI: 10.1002/cbf.70073
Rosmarinus officinalis Ethanolic Extracts Rescues BV-2 Cells via Modulating Inflammation and Redox Balance: Comparative Study With Carnosol and Carnosic Acid
Abstract
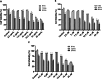
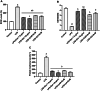
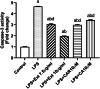
Neuroinflammation generally refers to an inflammatory response within the central nervous system caused by various pathological insults, including infection, trauma, ischemia, and toxins. As the brain's sentinel immune cell, microglia are tasked as the first responders to infection or tissue injury and initiating an inflammatory response. The perennial shrub plant Rosmarinus officinalis L. was reported to possess anti-inflammatory, anticancer, anti-nociceptive, antidiabetic, neuroprotective, and antioxidative properties. The present study aimed to investigate the effects of Rosmarinus officinalis ethanolic extracts on the lipopolysaccharide (LPS)-induced neuroinflammation model of BV-2 cells in comparison to carnosol and carnosic acid, phenolic diterpenes of the plant. Ultrasound-assisted extraction was used to have ethanolic extract of the plant. LPS was used to induce inflammation in BV-2 cells. Tumor necrosis alpha (TNF-α), interleukin 1 beta (IL-1β) secretion, reactive oxygen species (ROS) production, GSH/GSSG ratio, protein carbonyl level, and caspase-3 activity were evaluated. Inflammation induced by LPS was reduced by the ethanolic extract. Both carnosol and carnosic acid decreased the TNF-α and IL-1β levels as well. The ethanolic extract reduced ROS production and protein carbonylation, and increased GSH/GSSG ratio more effectively compared to the effects of carnosol and carnosic acid. Results depicted that caspase-3 activity was reduced by the ethanolic extract and this effect was more pronounced compared to carnosol and carnosic acid. The present study indicates the ethanolic extract of Rosmarinus officinalis rescues BV-2 cells from apoptosis via alleviating inflammation and oxidative stress.
Keywords: Rosmarinus officinalis L.; apoptosis; inflammation; lipopolysaccharide; microglia; redox modulation.
© 2025 The Author(s). Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
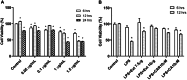
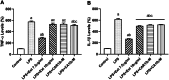
Similar articles
-
Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain.Biomed Pharmacother. 2017 Feb;86:441-449. doi: 10.1016/j.biopha.2016.12.049. Epub 2016 Dec 22. Biomed Pharmacother. 2017. PMID: 28012923
-
Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L.Fitoterapia. 2011 Apr;82(3):414-21. doi: 10.1016/j.fitote.2010.11.023. Epub 2010 Dec 1. Fitoterapia. 2011. PMID: 21129455
-
Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses.J Med Food. 2013 Apr;16(4):324-33. doi: 10.1089/jmf.2012.2577. Epub 2013 Mar 20. J Med Food. 2013. PMID: 23514231 Free PMC article.
-
Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation.Molecules. 2022 Jan 18;27(3):609. doi: 10.3390/molecules27030609. Molecules. 2022. PMID: 35163873 Free PMC article. Review.
-
Antidiabetic Effects and Mechanisms of Rosemary (Rosmarinus officinalis L.) and its Phenolic Components.Am J Chin Med. 2020;48(6):1353-1368. doi: 10.1142/S0192415X20500664. Am J Chin Med. 2020. PMID: 33016104 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

