Aged Gut Microbiota Contributes to Cognitive Impairment and Hippocampal Synapse Loss in Mice
- PMID: 40219707
- PMCID: PMC12266779
- DOI: 10.1111/acel.70064
Aged Gut Microbiota Contributes to Cognitive Impairment and Hippocampal Synapse Loss in Mice
Abstract
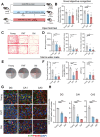
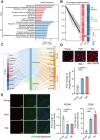
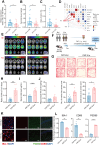
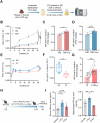
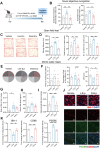
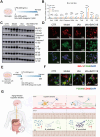
Gut microbiota alteration during the aging process serves as a causative factor for aging-related cognitive decline, which is characterized by the early hallmark, hippocampal synaptic loss. However, the impact and mechanistic role of gut microbiota in hippocampal synapse loss during aging remains unclear. Here, we observed that the fecal microbiota of naturally aged mice successfully transferred cognitive impairment and hippocampal synapse loss to young recipients. Multi-omics analysis revealed that aged gut microbiota was characterized with obvious change in Bifidobacterium pseudolongum (B.p) and metabolite of tryptophan, indoleacetic acid (IAA) in the periphery and brain. These features were also reproduced in young recipients that were transplanted with aged gut microbiota. Fecal B.p abundance was reduced in patients with cognitive impairment compared to healthy subjects and showed a positive correlation with cognitive scores. Microbiota transplantation from patients who had fewer B.p abundances yielded worse cognitive behavior in mice than those with higher B.p abundances. Meanwhile, supplementation of B.p was capable of producing IAA and enhancing peripheral and brain IAA bioavailability, as well as improving cognitive behaviors and microglia-mediated synapse loss in 5 × FAD transgenic mice. IAA produced from B.p was shown to prevent microglia engulfment of synapses in an aryl hydrocarbon receptor-dependent manner. This study reveals that aged gut microbiota -induced cognitive decline and microglia-mediated synapse loss that is, at least partially, due to the deficiency in B.p and its metabolite, IAA. It provides a proof-of-concept strategy for preventing neurodegenerative diseases by modulating gut probionts and their tryptophan metabolites.
Keywords: Bifidobacterium pseudolongum; aging‐related cognitive decline; microglia engulfment; synapse loss.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Gender Differences in Dendritic Damage, Gut Microbiota Dysbiosis, and Cognitive Impairment During Aging Processes.CNS Neurosci Ther. 2024 Dec;30(12):e70164. doi: 10.1111/cns.70164. CNS Neurosci Ther. 2024. PMID: 39723486 Free PMC article.
-
Gut microbiota-derived indoleacetic acid attenuates neuroinflammation and neurodegeneration in glaucoma through ahr/rage pathway.J Neuroinflammation. 2025 Jul 10;22(1):179. doi: 10.1186/s12974-025-03505-4. J Neuroinflammation. 2025. PMID: 40640940 Free PMC article.
-
Young gut microbiota transplantation improves the metabolic health of old mice.mSystems. 2025 Jun 17;10(6):e0160124. doi: 10.1128/msystems.01601-24. Epub 2025 May 30. mSystems. 2025. PMID: 40444969 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

