5-Methylcytosine methylation predicts cervical cancer prognosis, shaping immune cell infiltration
- PMID: 40219803
- PMCID: PMC12033582
- DOI: 10.1177/03000605251328301
5-Methylcytosine methylation predicts cervical cancer prognosis, shaping immune cell infiltration
Abstract
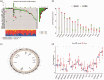
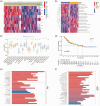
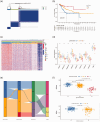
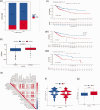
BackgroundEpigenetics, encompassing DNA and RNA modifications, has emerged as a prominent area of research in the post-genomic era. Numerous studies have elucidated the impact of epigenetics on tumor regulation. However, the methylation patterns of 5-methylcytosine in cervical cancer as well as its role in the tumor microenvironment and immunotherapy remain poorly understood.MethodsUtilizing a comprehensive dataset encompassing samples from 306 patients with cervical cancer from The Cancer Genome Atlas and Gene Expression Omnibus repositories, we conducted an in-depth analysis to evaluate the potential association between the modification patterns of 5-methylcytosine and the infiltration of cells within the tumor microenvironment, taking into account 11 regulators of 5-methylcytosine modification. Subsequently, we employed stepwise regression and Least Absolute Shrinkage and Selection Operator Cox regression to quantify 5-methylcytosine modification patterns in patients with cervical squamous cell carcinoma and endocervical adenocarcinoma, yielding the 5-methylcytosine score. Our study explored the link between the 5-methylcytosine score and clinical characteristics as well as prognostic outcomes in patients with cervical squamous cell carcinoma and endocervical adenocarcinoma.ResultsA comprehensive analysis of 306 patients with cervical cancer revealed two distinct 5-methylcytosine modification patterns, henceforth labeled as 5-methylcytosine clusters A and B. These clusters exhibited distinct immunological profiles and biological attributes, with 5-methylcytosine cluster A exhibiting a higher degree of immune cell infiltration. Utilizing univariate Cox regression analysis, we identified 367 genes regulated by 5-methylcytosine that were significantly correlated with patient prognosis. This analysis further stratified the samples into three distinct genomic subtypes. Survival analyses indicated that patients belonging to gene cluster C exhibited more favorable survival outcomes than those belonging to gene clusters A and B. Intriguingly, most 5-methylcytosine regulatory factors had higher expression levels in gene cluster B than in gene cluster A. Gene set enrichment analysis of a single sample revealed elevated immune cell infiltration within gene cluster B, indicating a stronger immune response in this cluster. The 5-methylcytosine score feature was utilized to determine the 5-methylcytosine modification pattern in cervical cancer, revealing that patients with low 5-methylcytosine scores exhibited better survival rates, whereas those with high scores had increased mutation frequencies and better treatment responses.ConclusionsThis research underscores the key role of 5-methylcytosine modification patterns in cervical cancer. Analysis of these patterns will deepen our understanding of the cervical cancer tumor microenvironment, paving the way for the development of more refined and effective immunotherapy strategies.
Keywords: 5-methylcytosine; Cervical cancer; RNA methylation; immunotherapy; tumor microenvironment.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Pan-Cancer Analysis of Immune Cell Infiltration Identifies a Prognostic Immune-Cell Characteristic Score (ICCS) in Lung Adenocarcinoma.Front Immunol. 2020 Jun 30;11:1218. doi: 10.3389/fimmu.2020.01218. eCollection 2020. Front Immunol. 2020. PMID: 32714316 Free PMC article.
-
N6-Methyladenosine-Related lncRNAs as potential biomarkers for predicting prognoses and immune responses in patients with cervical cancer.BMC Genom Data. 2022 Jan 18;23(1):8. doi: 10.1186/s12863-022-01024-2. BMC Genom Data. 2022. PMID: 35042477 Free PMC article.
-
m6A Regulator-Mediated Tumour Infiltration and Methylation Modification in Cervical Cancer Microenvironment.Front Immunol. 2022 Apr 29;13:888650. doi: 10.3389/fimmu.2022.888650. eCollection 2022. Front Immunol. 2022. PMID: 35572541 Free PMC article.
-
Tissue-resident immune cells in cervical cancer: emerging roles and therapeutic implications.Front Immunol. 2025 Apr 22;16:1541950. doi: 10.3389/fimmu.2025.1541950. eCollection 2025. Front Immunol. 2025. PMID: 40330461 Free PMC article. Review.
-
Research progress of DNA methylation in the diagnosis and treatment of thyroid carcinoma.Int Immunopharmacol. 2025 Apr 16;152:114426. doi: 10.1016/j.intimp.2025.114426. Epub 2025 Mar 9. Int Immunopharmacol. 2025. PMID: 40058105 Review.
Cited by
-
PTK6 mediated immune signatures revealed by single cell transcriptomic and multi omics big data analysis in cervical cancer.Discov Oncol. 2025 Aug 16;16(1):1566. doi: 10.1007/s12672-025-03365-7. Discov Oncol. 2025. PMID: 40818002 Free PMC article.
References
-
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001; 293: 1089–1093. - PubMed
-
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003; 33: 245–254. - PubMed
-
- Elit L, Fyles AW, Devries MC; Gynecology Cancer Disease Site Group et al.. Follow-up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol 2009; 114: 528–535. - PubMed

