β-hydroxybutyrate enhances brain metabolism in normoglycemia and hyperglycemia, providing cerebroprotection in a mouse stroke model
- PMID: 40219805
- PMCID: PMC11993551
- DOI: 10.1177/0271678X251334222
β-hydroxybutyrate enhances brain metabolism in normoglycemia and hyperglycemia, providing cerebroprotection in a mouse stroke model
Abstract
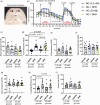
Hyperglycemia in poorly controlled diabetes is widely recognized as detrimental to organ dysfunction. However, the acute effects of hyperglycemia on brain metabolism and function are not fully understood. The potential protective benefit of ketone bodies on mitochondrial function in the brain has also not been well characterized. Here, we evaluated the acute effects of hyperglycemia and β-hydroxybutyrate (BHB) on brain metabolism by employing a novel approach leveraging adenosine triphosphate (ATP)-dependence of bioluminescence originating from luciferin-luciferase activity. Oxygen consumption rate was measured in ex vivo live brain punches to further evaluate mitochondrial function. Our data demonstrate that brain metabolism in mice is affected by acute exposure to high glucose. This short-term effect of glucose exposure was reduced by co-administration with the ketone body BHB. Additionally, we investigated the functional relevance of BHB using an in vivo photothrombotic stroke model to assess its cerebroprotective effects in presence or absence of acute hyperglycemia. BHB significantly reduced infarct size in the brain stroke model, providing functional evidence for its protective role in the brain. These findings suggest that BHB may effectively mitigate the adverse effects of metabolic stress and ischemic events on brain metabolism and function.
Keywords: Acute hyperglycemia; brain metabolism; in vivo brain imaging; oxygen consumption rate; β-hydroxybutyrate.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The funder, Astrocyte Pharmaceuticals, reviewed and approved the manuscript. JDL is co-founder and advisor for Astrocyte Pharmaceuticals. JDL is also an equity holder in Astrocyte. All other authors declare no competing interests or personal relationships that could influence the work reported in this paper.
Figures
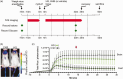

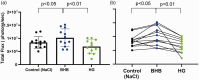
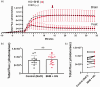
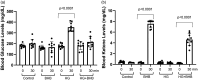

Update of
-
β-Hydroxybutyrate enhances brain metabolism in normoglycemia and hyperglycemia, providing cerebroprotection in a mouse stroke model.bioRxiv [Preprint]. 2025 Feb 24:2025.02.19.639087. doi: 10.1101/2025.02.19.639087. bioRxiv. 2025. Update in: J Cereb Blood Flow Metab. 2025 Apr 12:271678X251334222. doi: 10.1177/0271678X251334222. PMID: 40060617 Free PMC article. Updated. Preprint.
Similar articles
-
β-Hydroxybutyrate enhances brain metabolism in normoglycemia and hyperglycemia, providing cerebroprotection in a mouse stroke model.bioRxiv [Preprint]. 2025 Feb 24:2025.02.19.639087. doi: 10.1101/2025.02.19.639087. bioRxiv. 2025. Update in: J Cereb Blood Flow Metab. 2025 Apr 12:271678X251334222. doi: 10.1177/0271678X251334222. PMID: 40060617 Free PMC article. Updated. Preprint.
-
Imaging the Effects of β-Hydroxybutyrate on Peri-Infarct Neurovascular Function and Metabolism.Stroke. 2018 Sep;49(9):2173-2181. doi: 10.1161/STROKEAHA.118.020586. Stroke. 2018. PMID: 30354983
-
Is on-admission capillary blood beta-hydroxybutyrate concentration associated with the acute stroke severity and short-term functional outcome?Neurol Res. 2013 Nov;35(9):959-67. doi: 10.1179/1743132813Y.0000000239. Epub 2013 Jul 1. Neurol Res. 2013. PMID: 23815820
-
β-hydroxybutyrate and ischemic stroke: roles and mechanisms.Mol Brain. 2024 Jul 29;17(1):48. doi: 10.1186/s13041-024-01119-0. Mol Brain. 2024. PMID: 39075604 Free PMC article. Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
References
-
- Bueno NB, de Melo IS, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr 2013; 110: 1178–1187. - PubMed
-
- Bhanpuri NH, Hallberg SJ, Williams PT, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol 2018; 17: 56. - PMC - PubMed
-
- Gjuladin-Hellon T, Davies IG, Penson P, et al. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: a systematic review and meta-analysis. Nutr Rev 2019; 77: 161–180. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

