Deletion of microsomal epoxide hydrolase gene leads to increased density in cerebral vasculature and enhances cerebral blood flow in mice
- PMID: 40219924
- PMCID: PMC11994649
- DOI: 10.1177/0271678X251333234
Deletion of microsomal epoxide hydrolase gene leads to increased density in cerebral vasculature and enhances cerebral blood flow in mice
Abstract
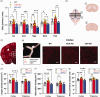
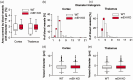
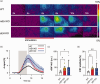
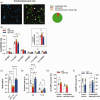
Microsomal epoxide hydrolase (mEH), first identified as detoxifying enzyme, can hydrolyze epoxyeicosatrienoic acids (EETs) to less active diols (DHETs). EETs are potent vasodilatory and pro-angiogenic lipids, also implicated in neurovascular coupling. In mouse brain, mEH is strongly expressed in vascular and perivascular cells in contrast to the related soluble epoxide hydrolase (sEH), predominantly found in astrocytes. While sEH inhibition in stroke has demonstrated neuroprotective effects and increases cerebral blood flow (CBF), data regarding the role of mEH in brain are scarce. Here, we explored the function of mEH in cerebral vasculature by comparing mEH-KO, sEH-KO and WT mice. Basal cerebral volume (CBV0) was significantly higher in various mEH-KO brain areas compared to WT and sEH-KO. In line, quantification of cerebral vasculature in cortex and thalamus revealed a higher capillary density in mEH-KO, but not in sEH-KO brain. Whisker-stimulated CBF changes were by factor two higher in both mEH-KO and sEH-KO. In acutely isolated cerebral endothelial cells the loss of mEH, but not of sEH, augmented total EET levels and decreased the DHET:EET ratio. Collectively, these data suggest an important function of mEH in the regulation of cerebral vasculature and activity-modulated CBF, presumably by controlling local levels of endothelial-derived EETs.
Keywords: Angiogenesis; EETs; cerebral blood flow; epoxide hydrolase; pericytes.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Beyond detoxification: a role for mouse mEH in the hepatic metabolism of endogenous lipids.Arch Toxicol. 2017 Nov;91(11):3571-3585. doi: 10.1007/s00204-017-2060-4. Epub 2017 Oct 3. Arch Toxicol. 2017. PMID: 28975360 Free PMC article.
-
Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism.Neuroscience. 2009 Oct 6;163(2):646-61. doi: 10.1016/j.neuroscience.2009.06.033. Epub 2009 Jun 18. Neuroscience. 2009. PMID: 19540314
-
Genetic enhancement of microsomal epoxide hydrolase improves metabolic detoxification but impairs cerebral blood flow regulation.Arch Toxicol. 2016 Dec;90(12):3017-3027. doi: 10.1007/s00204-016-1666-2. Epub 2016 Feb 2. Arch Toxicol. 2016. PMID: 26838043 Free PMC article.
-
Regulation of cardiovascular biology by microsomal epoxide hydrolase.Toxicol Res. 2021 Jan 21;37(3):285-292. doi: 10.1007/s43188-021-00088-z. eCollection 2021 Jul. Toxicol Res. 2021. PMID: 34295793 Free PMC article. Review.
-
Epoxyeicosatrienoic acids and soluble epoxide hydrolase in physiology and diseases of the central nervous system.Chin J Physiol. 2022 Jan-Feb;65(1):1-11. doi: 10.4103/cjp.cjp_80_21. Chin J Physiol. 2022. PMID: 35229747 Review.
References
-
- Oesch F. Significance of various enzymes in the control of reactive metabolites. Arch Toxicol 1987; 60: 174–178. - PubMed
-
- Marowsky A, Burgener J, Falck JR, et al.. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 2009; 163: 646–661. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

