Berberine bridge enzyme-like oxidases orchestrate homeostasis and signaling of oligogalacturonides in defense and upon mechanical damage
- PMID: 40220003
- PMCID: PMC11992967
- DOI: 10.1111/tpj.70150
Berberine bridge enzyme-like oxidases orchestrate homeostasis and signaling of oligogalacturonides in defense and upon mechanical damage
Abstract
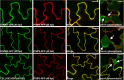
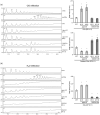
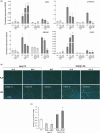
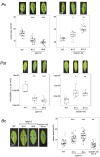
Plant immunity is triggered by endogenous elicitors known as damage-associated molecular patterns (DAMPs). Oligogalacturonides (OGs) are DAMPs released from the cell wall (CW) demethylated homogalacturonan during microbial colonization, mechanical or pest-provoked mechanical damage, and physiological CW remodeling. Berberine bridge enzyme-like (BBE-l) proteins named OG oxidases (OGOXs) oxidize and inactivate OGs to avoid deleterious growth-affecting hyper-immunity and possible cell death. Using OGOX1 over-expressing lines and ogox1/2 double mutants, we show that these enzymes determine the levels of active OGs vs. inactive oxidized products (ox-OGs). The ogox1/2-deficient plants have elevated levels of OGs, while plants overexpressing OGOX1 accumulate ox-OGs. The balance between OGs and ox-OGs affects disease resistance against Pseudomonas syringae pv. tomato, Pectobacterium carotovorum, and Botrytis cinerea depending on the microbial capacity to respond to OGs and metabolize ox-OGs. Gene expression upon plant infiltration with OGs reveals that OGOXs orchestrate OG signaling in defense as well as upon mechanical damage, pointing to these enzymes as apoplastic players in immunity and tissue repair.
Keywords: Damage‐Associated Molecular Patterns; berberine bridge enzyme‐like oxidases; oligogalacturonides; plant immunity.
© 2025 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Four Arabidopsis berberine bridge enzyme-like proteins are specific oxidases that inactivate the elicitor-active oligogalacturonides.Plant J. 2018 Apr;94(2):260-273. doi: 10.1111/tpj.13852. Epub 2018 Mar 15. Plant J. 2018. PMID: 29396998
-
An Arabidopsis berberine bridge enzyme-like protein specifically oxidizes cellulose oligomers and plays a role in immunity.Plant J. 2019 May;98(3):540-554. doi: 10.1111/tpj.14237. Epub 2019 Mar 7. Plant J. 2019. PMID: 30664296
-
Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5533-8. doi: 10.1073/pnas.1504154112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870275 Free PMC article.
-
Dampening the DAMPs: How Plants Maintain the Homeostasis of Cell Wall Molecular Patterns and Avoid Hyper-Immunity.Front Plant Sci. 2020 Dec 17;11:613259. doi: 10.3389/fpls.2020.613259. eCollection 2020. Front Plant Sci. 2020. PMID: 33391327 Free PMC article. Review.
-
Cell wall bricks of defence: the case study of oligogalacturonides.Front Plant Sci. 2025 Mar 25;16:1552926. doi: 10.3389/fpls.2025.1552926. eCollection 2025. Front Plant Sci. 2025. PMID: 40201780 Free PMC article. Review.
References
-
- Anderson, C.M. , Wagner, T.A. , Perret, M. , He, Z.H. , He, D. & Kohorn, B.D. (2001) WAKs: cell wall‐associated kinases linking the cytoplasm to the extracellular matrix. Plant Molecular Biology, 47, 197–206. - PubMed
-
- Bednarek, P. , Pislewska‐Bednarek, M. , Svatos, A. , Schneider, B. , Doubsky, J. , Mansurova, M. et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad‐spectrum antifungal defense. Science, 323, 101–106. - PubMed
-
- Benedetti, M. , Mattei, B. , Pontiggia, D. , Salvi, G. , Savatin, D.V. & Ferrari, S. (2017) Methods of isolation and characterization of oligogalacturonide elicitors. Methods in Molecular Biology, 1578, 25–38. - PubMed
-
- Benedetti, M. , Pontiggia, D. , Raggi, S. , Cheng, Z. , Scaloni, F. , Ferrari, S. et al. (2015) Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage‐associated molecular patterns. Proceedings of the National Academy of Sciences of the United States of America, 112(17), 5533–5538. Available from: 10.1073/pnas.1504154112 - DOI - PMC - PubMed
-
- Benedetti, M. , Verrascina, I. , Pontiggia, D. , Locci, F. , Mattei, B. , De Lorenzo, G. et al. (2018) Four Arabidopsis berberine bridge enzyme‐like proteins are specific oxidases that inactivate the elicitor‐active oligogalacturonides. The Plant Journal, 94, 260–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

